Establishment of a Novel Humanized Mouse Model To Investigate In Vivo Activation and Depletion of Patient-Derived HIV Latent Reservoirs
- PMID: 30626677
- PMCID: PMC6401459
- DOI: 10.1128/JVI.02051-18
Establishment of a Novel Humanized Mouse Model To Investigate In Vivo Activation and Depletion of Patient-Derived HIV Latent Reservoirs
Abstract
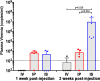
Curing HIV infection has been thwarted by the persistent reservoir of latently infected CD4+ T cells, which reinitiate systemic infection after antiretroviral therapy (ART) interruption. To evaluate reservoir depletion strategies, we developed a novel preclinical in vivo model consisting of immunodeficient mice intrasplenically injected with peripheral blood mononuclear cells (PBMC) from long-term ART-suppressed HIV-infected donors. In the absence of ART, these mice developed rebound viremia which, 2 weeks after PBMC injection, was 1,000-fold higher (mean = 9,229,281 HIV copies/ml) in mice injected intrasplenically than in mice injected intraperitoneally (mean = 6,838 HIV copies/ml) or intravenously (mean = 591 HIV copies/ml). One week after intrasplenic PBMC injection, in situ hybridization of the spleen demonstrated extensive disseminated HIV infection, likely initiated from in vivo-reactivated primary latently infected cells. The time to viremia was delayed significantly by treatment with a broadly neutralizing antibody, 10-1074, compared to treatment with 10-1074-FcRnull, suggesting that 10-1074 mobilized Fc-mediated effector mechanisms to deplete the replication-competent reservoir. This was supported by phylogenetic analysis of Env sequences from viral-outgrowth cultures and untreated, 10-1074-treated, or 10-1074-FcRnull-treated mice. The predominant sequence cluster detected in viral-outgrowth cultures and untreated mouse plasma was significantly reduced in the plasma of 10-1074-treated mice, whereas two new clusters emerged that were not detected in viral-outgrowth cultures or plasma from untreated mice. These new clusters lacked mutations associated with 10-1074 resistance. Taken together, these data indicated that 10-1074 treatment depletes the reservoir of latently infected cells harboring replication competent HIV. Furthermore, this mouse model represents a new in vivo approach for the preclinical evaluation of new HIV cure strategies.IMPORTANCE Sustained remission of HIV infection is prevented by a persistent reservoir of latently infected cells capable of reinitiating systemic infection and viremia. To evaluate strategies to reactivate and deplete this reservoir, we developed and characterized a new humanized mouse model consisting of highly immunodeficient mice intrasplenically injected with peripheral blood mononuclear cells from long-term ART-suppressed HIV-infected donors. Reactivation and dissemination of HIV infection was visualized in the mouse spleens in parallel with the onset of viremia. The applicability of this model for evaluating reservoir depletion treatments was demonstrated by establishing, through delayed time to viremia and phylogenetic analysis of plasma virus, that treatment of these humanized mice with a broadly neutralizing antibody, 10-1074, depleted the patient-derived population of latently infected cells. This mouse model represents a new in vivo approach for the preclinical evaluation of new HIV cure strategies.
Keywords: HIV; latent infection; reservoir.
Copyright © 2019 American Society for Microbiology.
Figures






Similar articles
-
Antibody-Mediated CD4 Depletion Induces Homeostatic CD4+ T Cell Proliferation without Detectable Virus Reactivation in Antiretroviral Therapy-Treated Simian Immunodeficiency Virus-Infected Macaques.J Virol. 2018 Oct 29;92(22):e01235-18. doi: 10.1128/JVI.01235-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30185596 Free PMC article.
-
Potent In Vivo NK Cell-Mediated Elimination of HIV-1-Infected Cells Mobilized by a gp120-Bispecific and Hexavalent Broadly Neutralizing Fusion Protein.J Virol. 2017 Sep 27;91(20):e00937-17. doi: 10.1128/JVI.00937-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28794022 Free PMC article.
-
Humanized Mouse Model of HIV-1 Latency with Enrichment of Latent Virus in PD-1+ and TIGIT+ CD4 T Cells.J Virol. 2019 May 1;93(10):e02086-18. doi: 10.1128/JVI.02086-18. Print 2019 May 15. J Virol. 2019. PMID: 30842333 Free PMC article.
-
Humanized mouse models of latent HIV infection.Curr Opin Virol. 2017 Aug;25:97-104. doi: 10.1016/j.coviro.2017.07.027. Epub 2017 Aug 12. Curr Opin Virol. 2017. PMID: 28810166 Review.
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
Cited by
-
Antibodies for Human Immunodeficiency Virus-1 Cure Strategies.J Infect Dis. 2021 Feb 15;223(12 Suppl 2):22-31. doi: 10.1093/infdis/jiaa165. J Infect Dis. 2021. PMID: 33586772 Free PMC article. Review.
-
μ-Lat: A mouse model to evaluate human immunodeficiency virus eradication strategies.FASEB J. 2020 Nov;34(11):14615-14630. doi: 10.1096/fj.202001612RR. Epub 2020 Sep 9. FASEB J. 2020. PMID: 32901981 Free PMC article.
-
Baseline and time-updated factors in preclinical development of anionic dendrimers as successful anti-HIV-1 vaginal microbicides.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 May;14(3):e1774. doi: 10.1002/wnan.1774. Epub 2022 Jan 12. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022. PMID: 35018739 Free PMC article. Review.
-
Melanoma in people living with HIV: Immune landscape dynamics and the role of immuno- and antiviral therapies.Cancer Metastasis Rev. 2024 Nov 29;44(1):9. doi: 10.1007/s10555-024-10230-6. Cancer Metastasis Rev. 2024. PMID: 39609320 Free PMC article. Review.
-
T cell receptor-targeted immunotherapeutics drive selective in vivo HIV- and CMV-specific T cell expansion in humanized mice.J Clin Invest. 2021 Dec 1;131(23):e141051. doi: 10.1172/JCI141051. J Clin Invest. 2021. PMID: 34673568 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

