Nutrient regulation of signaling and transcription
- PMID: 30626734
- PMCID: PMC6378989
- DOI: 10.1074/jbc.AW119.003226
Nutrient regulation of signaling and transcription
Abstract
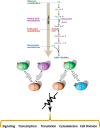
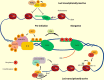
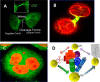
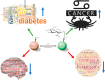
In the early 1980s, while using purified glycosyltransferases to probe glycan structures on surfaces of living cells in the murine immune system, we discovered a novel form of serine/threonine protein glycosylation (O-linked β-GlcNAc; O-GlcNAc) that occurs on thousands of proteins within the nucleus, cytoplasm, and mitochondria. Prior to this discovery, it was dogma that protein glycosylation was restricted to the luminal compartments of the secretory pathway and on extracellular domains of membrane and secretory proteins. Work in the last 3 decades from several laboratories has shown that O-GlcNAc cycling serves as a nutrient sensor to regulate signaling, transcription, mitochondrial activity, and cytoskeletal functions. O-GlcNAc also has extensive cross-talk with phosphorylation, not only at the same or proximal sites on polypeptides, but also by regulating each other's enzymes that catalyze cycling of the modifications. O-GlcNAc is generally not elongated or modified. It cycles on and off polypeptides in a time scale similar to phosphorylation, and both the enzyme that adds O-GlcNAc, the O-GlcNAc transferase (OGT), and the enzyme that removes O-GlcNAc, O-GlcNAcase (OGA), are highly conserved from C. elegans to humans. Both O-GlcNAc cycling enzymes are essential in mammals and plants. Due to O-GlcNAc's fundamental roles as a nutrient and stress sensor, it plays an important role in the etiologies of chronic diseases of aging, including diabetes, cancer, and neurodegenerative disease. This review will present an overview of our current understanding of O-GlcNAc's regulation, functions, and roles in chronic diseases of aging.
Keywords: Alzheimer's disease; O-GlcNAcase; O-GlcNAcylation; O-linked N-acetylglucosamine (O-GlcNAc); O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT); cancer; diabetes; kinases; neurodegeneration; phosphorylation.
© 2019 Hart.
Conflict of interest statement
The author declares that he has no conflicts of interest with the contents of this article
Figures
References
-
- Torres C. R., and Hart G. W. (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes: evidence for O-linked GlcNAc. J. Biol. Chem. 259, 3308–3317 - PubMed
-
- Holt G. D., and Hart G. W. (1986) The subcellular distribution of terminal N-acetylglucosamine moieties: localization of a novel protein-saccharide linkage, O-linked GlcNAc. J. Biol. Chem. 261, 8049–8057 - PubMed
-
- Holt G. D., Haltiwanger R. S., Torres C. R., and Hart G. W. (1987) Erythrocytes contain cytoplasmic glycoproteins: O-linked GlcNAc on Band 4.1. J. Biol. Chem. 262, 14847–14850 - PubMed
-
- Hanover J. A., Cohen C. K., Willingham M. C., and Park M. K. (1987) O-Linked N-acetylglucosamine is attached to proteins of the nuclear pore: evidence for cytoplasmic and nucleoplasmic glycoproteins. J. Biol. Chem. 262, 9887–9894 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

