Metabolic and microstructural alterations in the SLE brain correlate with cognitive impairment
- PMID: 30626758
- PMCID: PMC6485360
- DOI: 10.1172/jci.insight.124002
Metabolic and microstructural alterations in the SLE brain correlate with cognitive impairment
Abstract
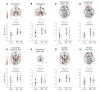
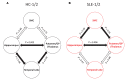
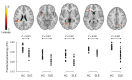
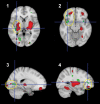
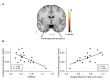
To address challenges in the diagnosis of cognitive dysfunction (CD) related to systemic lupus erythematosus-associated (SLE-associated) autoimmune mechanisms rather than confounding factors, we employed an integrated approach, using resting-state functional (FDG-PET) and structural (diffusion tensor imaging [DTI]) neuroimaging techniques and cognitive testing, in adult SLE patients with quiescent disease and no history of neuropsychiatric illness. We identified resting hypermetabolism in the sensorimotor cortex, occipital lobe, and temporal lobe of SLE subjects, in addition to validation of previously published resting hypermetabolism in the hippocampus, orbitofrontal cortex, and putamen/GP/thalamus. Regional hypermetabolism demonstrated abnormal interregional metabolic correlations, associated with impaired cognitive performance, and was stable over 15 months. DTI analyses demonstrated 4 clusters of decreased microstructural integrity in white matter tracts adjacent to hypermetabolic regions and significantly diminished connecting tracts in SLE subjects. Decreased microstructural integrity in the parahippocampal gyrus correlated with impaired spatial memory and increased serum titers of DNRAb, a neurotoxic autoantibody associated with neuropsychiatric lupus. These findings of regional hypermetabolism, associated with decreased microstructural integrity and poor cognitive performance and not associated with disease duration, disease activity, medications, or comorbid disease, suggest that this is a reproducible, stable marker for SLE-associated CD that may be may be used for early disease detection and to discriminate between groups, evaluate response to treatment strategies, or assess disease progression.
Keywords: Autoimmune diseases; Autoimmunity; Neuroimaging; Neuroscience.
Conflict of interest statement
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

