Dominance of vaccine serotypes in pediatric invasive pneumococcal infections in Portugal (2012-2015)
- PMID: 30626918
- PMCID: PMC6327022
- DOI: 10.1038/s41598-018-36799-x
Dominance of vaccine serotypes in pediatric invasive pneumococcal infections in Portugal (2012-2015)
Abstract
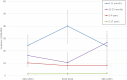
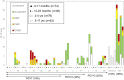
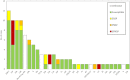
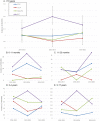
We evaluated the impact of continued 13-valent pneumococcal conjugate vaccine (PCV13) use in the private market (uptake of 61%) in pediatric invasive pneumococcal disease (pIPD) in Portugal (2012-2015). The most frequently detected serotypes were: 3 (n = 32, 13.8%), 14 (n = 23, 9.9%), 1 (n = 23, 9.9%), 7F (n = 15, 6.4%), 19A (n = 13, 5.6%), 6B and 15B/C (both n = 12, 5.2%), and 24F, 10A and 12B (all with n = 10, 4.3%). Taken together, non-PCV13 serotypes were responsible for 42.2% of pIPD with a known serotype. The use of PCR to detect and serotype pneumococci in both pleural and cerebrospinal fluid samples contributed to 18.1% (n = 47) of all pIPD. Serotype 3 was mostly detected by PCR (n = 21/32, 65.6%) and resulted from a relevant number of vaccine failures. The incidence of pIPD varied in the different age groups but without a clear trend. There were no obvious declines of the incidence of pIPD due to serotypes included in any of the PCVs, and PCV13 serotypes still accounted for the majority of pIPD (57.8%). Our study indicates that a higher vaccination uptake may be necessary to realize the full benefits of PCVs, even after 15 years of moderate use, and highlights the importance of using molecular methods in pIPD surveillance, since these can lead to substantially increased case ascertainment and identification of particular serotypes as causes of pIPD.
Conflict of interest statement
J.M.C. has received research grants administered through his university and received honoraria for serving on the speakers bureaus of Pfizer and Merck Sharp and Dohme. M.R. has received honoraria for serving on the speakers bureau of Pfizer and for consulting for GlaxoSmithKline and Merck Sharp and Dohme. The other authors declare no conflict of interest. No company or financing body had any interference in the decision to publish.
Figures
References
-
- Aguiar, S. I. et al. Decreasing incidence and changes in serotype distribution of invasive pneumococcal disease in persons aged under 18 years since introduction of 10-valent and 13-valent conjugate vaccines in Portugal, July 2008 to June 2012. Euro Surveill. 19, 10.2807/1560-7917.ES2014.19.12.20750 (2014). - PubMed
-
- Aguiar SI, Serrano I, Pinto FR, Melo-Cristino J, Ramirez M. Changes in Streptococcus pneumoniae serotypes causing invasive disease with non-universal vaccination coverage of the seven-valent conjugate vaccine. Clin. Microbiol. Infect. 2008;14:835–843. doi: 10.1111/j.1469-0691.2008.02031.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

