SEOM clinical guidelines on cardiovascular toxicity (2018)
- PMID: 30627982
- PMCID: PMC6339681
- DOI: 10.1007/s12094-018-02017-3
SEOM clinical guidelines on cardiovascular toxicity (2018)
Abstract
One of the most common side effects of cancer treatment is cardiovascular disease, which substantially impacts long-term survivor's prognosis. Cardiotoxicity can be related with either a direct side effect of antitumor therapies or an accelerated development of cardiovascular diseases in the presence of preexisting risk factors. Even though it is widely recognized as an alarming clinical problem, scientific evidence is scarce in the management of these complications in cancer patients. Consequently, current recommendations are based on expert consensus. This Guideline represents SEOM's ongoing commitment to progressing and improving supportive care for cancer patients.
Keywords: Cancer; Cardiotoxicity; Chemotherapy; Early detection; Risk assessment.
Conflict of interest statement
Conflict of interest
JAV has nothing to disclose. AMG has nothing to disclose. RDP has nothing to disclose. AS reports speaker honoraria from Roche, Pfizer, Astra Zeneca, Novartis, MSD and Pierre Fabre and Advisory Board from Roche, Tesaro, Clovis, Astra Zeneca and Novartis, outside the submitted work has nothing to disclose. RA has nothing to disclose. CB reports grants from LEO Pharma, Roche, MSD, ROVI, Sanofi, BMS and Mylan. Has received speaker honoraria from Esteve and Kyowa Kirin and Advisory boards from Kyowa kirin, Omakase and Roche, outside the submitted work. SC has nothing to disclose. JG reports speaker honoraria from Roche, Pfizer and Novartis, and Advisory board from Roche and Novartis, outside the submitted work. SG reports advisory boards from AstraZeneca, Celgene, Roche y Novartis. TL reports honoraria for teaching from Janssen-Cilag, Teva, Gilead, Daiichi, Novartis and Pfizer, outside the submitted work.
Ethical standards
The current study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
For this type of study formal consent is not required.
Figures
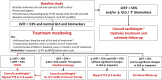



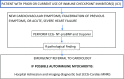
Comment in
-
Some remarks to SEOM clinical guidelines on cardiovascular toxicity (2018).Clin Transl Oncol. 2019 Dec;21(12):1786-1787. doi: 10.1007/s12094-019-02135-6. Epub 2019 May 29. Clin Transl Oncol. 2019. PMID: 31144212 No abstract available.
References
-
- Zamorano JL, Lancellotti P, Rodríguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211. - DOI - PubMed

