A comprehensive study of immunology repertoires in both preoperative stage and postoperative stage in patients with colorectal cancer
- PMID: 30628178
- PMCID: PMC6418368
- DOI: 10.1002/mgg3.504
A comprehensive study of immunology repertoires in both preoperative stage and postoperative stage in patients with colorectal cancer
Abstract
Background: Colorectal cancer (CRC) is the 3rd most common cancer type in the world. The correlation between immune repertoire and prognosis of CRC has been well studied in the last decades. The diversity and stability of the immune cells can be measured by hypervariable complementarity-determining region 3 (CDR3) segments of the T-cell receptor (TCR).
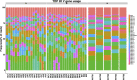
Methods: In this study, we collected five healthy controls and 19 CRC patients' peripheral blood mononuclear cells (PBMCs) in three stages, namely 1 day preoperative, 3 days' postoperative, and 7 days' postoperative, respectively. Simultaneously, we have also done the comparative analysis of these two different anesthesia methods, namely TIVA and CEGA. Sequencing of the TCR segments has been performed by multiplex PCR and high-throughput next-generation sequencing. We also analyzed the distribution of CDR3 length, highly expansion clones (HECs), TRBV, and TRBJ gene usage.
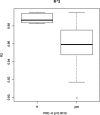
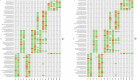
Results: Our result showed a significant difference between TCR CDR3 length distribution and HEC distribution between CRC patients and healthy controls. We also found that TRBV11-2, TRBV12-1, TRBV16, TRBV3-2, TRBV4-2, TRBV4-3, TRBV5-4, TRBV6-8, TRBV7-8, TRBV7-9 and RBV11-2, TRBV12-1, TRBV16, TRBV3-2, TRBV4-2, TRBV4-3, TRBV5-4, TRBV6-8, TRBV7-8, and TRBV7-9 usages are different between CRC patients and healthy controls.
Conclusion: In conclusion, CRC patients were presented with different immune repertoire in comparison with healthy controls. In this study, significant difference in TRBV and TRBJ gene usage in between case and control group could provide some potential biomarker for the diagnosis and the treatment of the patients with CRC.
Keywords: CDR3; CEGA; CRC; TCRs; TIVA; repertoire.
© 2019 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflict of interests to declare.
Figures




Similar articles
-
T-cell receptor dynamics in digestive system cancers: a multi-layer machine learning approach for tumor diagnosis and staging.Front Immunol. 2025 Apr 8;16:1556165. doi: 10.3389/fimmu.2025.1556165. eCollection 2025. Front Immunol. 2025. PMID: 40264789 Free PMC article.
-
High-throughput T cell receptor sequencing reveals differential immune repertoires in autoimmune thyroid diseases.Mol Cell Endocrinol. 2022 Jun 15;550:111644. doi: 10.1016/j.mce.2022.111644. Epub 2022 Apr 13. Mol Cell Endocrinol. 2022. PMID: 35429598
-
Primary exploration of CDR3 spectratyping and molecular features of TCR β chain in the peripheral blood and tissue of patients with colorectal carcinoma.Cancer Epidemiol. 2010 Dec;34(6):733-40. doi: 10.1016/j.canep.2010.06.010. Epub 2010 Jul 31. Cancer Epidemiol. 2010. PMID: 20674537
-
Study of the T-cell receptor repertoire by CDR3 spectratyping.J Immunol Methods. 2017 Jan;440:1-11. doi: 10.1016/j.jim.2016.11.001. Epub 2016 Nov 5. J Immunol Methods. 2017. PMID: 27823906 Review.
-
Current status and recent advances of next generation sequencing techniques in immunological repertoire.Genes Immun. 2016 Apr;17(3):153-64. doi: 10.1038/gene.2016.9. Epub 2016 Mar 10. Genes Immun. 2016. PMID: 26963138 Review.
Cited by
-
Cancer neoantigens as potential targets for immunotherapy.Clin Exp Metastasis. 2022 Feb;39(1):51-60. doi: 10.1007/s10585-021-10091-1. Epub 2021 May 5. Clin Exp Metastasis. 2022. PMID: 33950415 Free PMC article. Review.
-
T-cell Receptor Repertoire Analysis in the Context of Transarterial Chemoembolization Synergy with Systemic Therapy for Hepatocellular Carcinoma.J Clin Transl Hepatol. 2025 Jan 28;13(1):69-83. doi: 10.14218/JCTH.2024.00238. Epub 2024 Nov 12. J Clin Transl Hepatol. 2025. PMID: 39801788 Free PMC article. Review.
-
Identification of LncRNA Prognostic Markers for Ovarian Cancer by Integration of Co-expression and CeRNA Network.Front Genet. 2021 Feb 16;11:566497. doi: 10.3389/fgene.2020.566497. eCollection 2020. Front Genet. 2021. PMID: 33664764 Free PMC article.
-
Evaluating the Potential of T Cell Receptor Repertoires in Predicting the Prognosis of Resectable Non-Small Cell Lung Cancers.Mol Ther Methods Clin Dev. 2020 May 22;18:73-83. doi: 10.1016/j.omtm.2020.05.020. eCollection 2020 Sep 11. Mol Ther Methods Clin Dev. 2020. PMID: 32995352 Free PMC article.
-
Biomarkers of Response and Resistance to Immunotherapy in Microsatellite Stable Colorectal Cancer: Toward a New Personalized Medicine.Cancers (Basel). 2022 Apr 29;14(9):2241. doi: 10.3390/cancers14092241. Cancers (Basel). 2022. PMID: 35565369 Free PMC article. Review.
References
-
- Adelson, P. , Fusco, K. , Karapetis, C. , Wattchow, D. , Joshi, R. , Price, T. , & Roder, D. (2018). Use of guideline‐recommended adjuvant therapies and survival outcomes for people with colorectal cancer at tertiary referral hospitals in South Australia. Journal of Evaluation in Clinical Practice, 24(1), 135–144. 10.1111/jep.12757 - DOI - PubMed
-
- Chen, Y. , Xu, Y. , Zhao, M. , Liu, Y. , Gong, M. , Xie, C. , … Wang, Z. (2016a). High‐throughput T cell receptor sequencing reveals distinct repertoires between tumor and adjacent non‐tumor tissues in HBV‐associated HCC. Oncoimmunology, 5, e1219010 10.1080/2162402X.2016.1219010 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

