Early treatment of acute hepatitis C infection is cost-effective in HIV-infected men-who-have-sex-with-men
- PMID: 30629662
- PMCID: PMC6328146
- DOI: 10.1371/journal.pone.0210179
Early treatment of acute hepatitis C infection is cost-effective in HIV-infected men-who-have-sex-with-men
Abstract
Background: Treatment of hepatitis C virus infections (HCV) with direct acting antivirals (DAA) can prevent new infections since cured individuals cannot transmit HCV. However, as DAAs are expensive, many countries defer treatment to advances stages of fibrosis, which results in ongoing transmission. We assessed the epidemiological impact and cost-effectiveness of treatment initiation in different stages of infection in the Netherlands where the epidemic is mainly concentrated among HIV-infected MSMs.
Methods: We calibrated a deterministic mathematical model to the Dutch HCV epidemic among HIV-infected MSM to compare three different DAA treatment scenarios: 1) immediate treatment, 2) treatment delayed to chronic infection allowing spontaneous clearance to occur, 3) treatment delayed until F2 fibrosis stage. All scenarios are simulated from 2015 onwards. Total costs, quality adjusted life years (QALY), incremental cost-effectiveness ratios (ICERs), and epidemiological impact were calculated from a providers perspective over a lifetime horizon. We used a DAA price of €35,000 and 3% discounting rates for cost and QALYs.
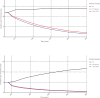
Results: Immediate DAA treatment lowers the incidence from 1.2/100 person-years to 0.2/100 person-years (interquartile range 0.1-0.2) and the prevalence from 5.0/100 person-years to 0.5/100 person-years (0.4-0.6) after 20 years. Delayed treatment awaiting spontaneous clearance will result in a similar reduction. However, further delayed treatment to F2 will increases the incidence and prevalence. Earlier treatment will cost society €68.3 and €75.1 million over a lifetime for immediate and awaiting until the chronic stage, respectively. The cost will increase if treatment is further delayed until F2 to €98.4 million. Immediate treatment will prevent 7070 new infections and gains 3419 (3019-3854) QALYs compared to F2 treatment resulting in a cost saving ICER. Treatment in the chronic stage is however dominated.
Conclusions: Early DAA treatment for HIV-infected MSM is an excellent and sustainable tool to meet the WHO goal of eliminating HCV in 2030.
Conflict of interest statement
Stephanie Popping: reports funding in the form of a unrestricted educational grant by Gilead Sciences [NL-2018-000171] and grants from Gilead [215001269], MSD [SDD 343462], ViiV Healthcare [14-0614-ViiV], and Janssen [771290]. Sebastiaan J. Hullegie: reports grants from Merck [MSD IISA 11/jul/2015], during the conduct of the study; non-financial support from Gilead, non-financial support from MSD, outside the submitted work Anne Boerekamps: reports no conflict of interest Bart J.A. Rijnders: reports grants from MSD [MSD IISA 11/jul/2015], Gilead [IN-NL-987- 4558/4652/4653] and honoraria from Jansen-Cilag, BMS, Pfizer and Viiv Robert J. de Knegt: Reports honoraria for consulting or speaking (last 5 years): AbbVie, BMS, Gilead, Janssen-Cilag, Merck/MSD, Roche. Jürgen K. Rockstroh: reports honaria for lectures and/or consultancies from Abbott, AbbVie, Bionor, BMS, Cipla, Gilead, Janssen, Merck, Roche, Viiv A.Verbon: reports no conflict of interest, Charles A.B. Boucher: reports grants from Gilead sciences [NL-2018-000171] and [215001269], MSD [SDD 343462], ViiV Healthcare [14-0614-ViiV], Janssen [771290], and Boehringer [S14064/32844]]. Brooke E. Nichols: no conflict of interest, David A.M.C. van de Vijver: reports grants from Gilead sciences [NL-2018-000171] and [215001269], MSD [SDD 343462], ViiV Healthcare [14-0614-ViiV], and Janssen [771290]. None of these competing interests alter our adherence to PLOS ONE policies on sharing data and materials. We confirm that none of the funders had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures

References
-
- Boerekamps A, van den Berk GE, Lauw FN, Leyten EM, van Kasteren ME, van Eeden A, et al. Declining Hepatitis C Virus (HCV) Incidence in Dutch Human Immunodeficiency Virus-Positive Men Who Have Sex With Men After Unrestricted Access to HCV Therapy. Clin Infect Dis. 2018;66(9):1360–5. 10.1093/cid/cix1007 - DOI - PubMed
-
- Global Hepatitis Report 2017. Geneva: World Health Organization; 2017. Geneva: 2017 CC BY-NC-SA 3.0 IGO.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

