Development of a Human Antibody Cocktail that Deploys Multiple Functions to Confer Pan-Ebolavirus Protection
- PMID: 30629917
- PMCID: PMC6396299
- DOI: 10.1016/j.chom.2018.12.004
Development of a Human Antibody Cocktail that Deploys Multiple Functions to Confer Pan-Ebolavirus Protection
Abstract
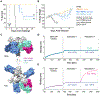
Passive administration of monoclonal antibodies (mAbs) is a promising therapeutic approach for Ebola virus disease (EVD). However, all mAbs and mAb cocktails that have entered clinical development are specific for a single member of the Ebolavirus genus, Ebola virus (EBOV), and ineffective against outbreak-causing Bundibugyo virus (BDBV) and Sudan virus (SUDV). Here, we advance MBP134, a cocktail of two broadly neutralizing human mAbs, ADI-15878 from an EVD survivor and ADI-23774 from the same survivor but specificity-matured for SUDV GP binding affinity, as a candidate pan-ebolavirus therapeutic. MBP134 potently neutralized all ebolaviruses and demonstrated greater protective efficacy than ADI-15878 alone in EBOV-challenged guinea pigs. A second-generation cocktail, MBP134AF, engineered to effectively harness natural killer (NK) cells afforded additional improvement relative to its precursor in protective efficacy against EBOV and SUDV in guinea pigs. MBP134AF is an optimized mAb cocktail suitable for evaluation as a pan-ebolavirus therapeutic in nonhuman primates.
Keywords: Ebola glycoprotein; Ebola virus; antibody cocktail; antibody engineering; antiviral drug; broadly neutralizing antibodies; ebolavirus; filovirus; human monoclonal antibodies; immunotherapy.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures




Comment in
-
It Takes a Matured mAb to Treat Ebolavirus Infection.Cell Host Microbe. 2019 Jan 9;25(1):10-12. doi: 10.1016/j.chom.2018.12.013. Cell Host Microbe. 2019. PMID: 30629912
References
-
- Brannan JM, Froude JW, Prugar LI, Bakken RR, Zak SE, Daye SP, Wilhelmsen CE, and Dye JM (2015). Interferon α/β Receptor-Deficient Mice as a Model for Ebola Virus Disease. J. Infect. Dis 212 Suppl 2, S282–94. - PubMed
-
- Bray M, Davis K, Geisbert T, Schmaljohn C, and Huggins J (1998). A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis 178, 651–661. - PubMed
-
- Chao G, Lau WL, Hackel BJ, Sazinsky SL, Lippow SM, and Wittrup KD (2006). Isolating and engineering human antibodies using yeast surface display. Nat. Protoc 1, 755–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

