A role for N-acetylaspartylglutamate (NAAG) and mGluR3 in cognition
- PMID: 30630041
- PMCID: PMC6397785
- DOI: 10.1016/j.nlm.2019.01.006
A role for N-acetylaspartylglutamate (NAAG) and mGluR3 in cognition
Abstract
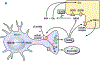
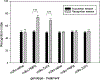
The peptide transmitter N-acetylaspartylglutamate (NAAG) and its receptor, the type 3 metabotropic glutamate receptor (mGluR3, GRM3), are prevalent and widely distributed in the mammalian nervous system. Drugs that inhibit the inactivation of synaptically released NAAG have procognitive activity in object recognition and other behavioral models. These inhibitors also reverse cognitive deficits in animal models of clinical disorders. Antagonists of mGluR3 block these actions and mice that are null mutant for this receptor are insensitive to the actions of these procognitive drugs. A positive allosteric modulator of this receptor also has procognitive activity. While some data suggest that drugs acting on mGluR3 achieve their procognitive action by increasing arousal during acquisition training, exploration of the procognitive efficacy of NAAG is in its early stages and thus substantial opportunities exist to define the breadth and nature of this activity.
Keywords: Glutamate carboxypeptidase II; Memory; Metabotropic receptors; N-acetylaspartylglutamate; NAAG; mGluR3.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
References
-
- Allen K,D, Regier M,J, Hsieh C, Tsokas P, Barnard M, Phatarpekar S, Wolk J, Sacktor T,C, Fenton A,A & Hernández A,I (2018) Learning-induced ribosomal RNA is required for memory consolidation in mice-Evidence of differentially expressed rRNA variants in learning and memory. PLoS One, 13(10), e0203374. - PMC - PubMed
-
- Altinbilek B & Manahan-Vaughan D (2009) A specific role for group II metabotropic glutamate receptors in hippocampal long-term depression and spatial memory. Neuroscience, 158(1), 149–158. - PubMed
-
- Bishop JR, Reilly JL, Harris MS, Patel SR, Kittles R, Badner JA, Prasad KM, Nimgaonkar VL, Keshavan MS & Sweeney JA (2015) Pharmacogenetic associations of the type-3 metabotropic glutamate receptor (GRM3) gene with working memory and clinical symptom response to antipsychotics in first-episode schizophrenia. Psychopharmacology (Berl), 232(1), 145–154. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

