Multiscale characterization of heart failure
- PMID: 30630123
- PMCID: PMC6369012
- DOI: 10.1016/j.actbio.2018.12.053
Multiscale characterization of heart failure
Abstract
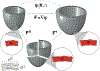
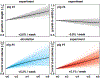
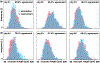
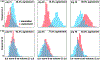
Dilated cardiomyopathy is a progressive irreversible disease associated with contractile dysfunction and heart failure. During dilated cardiomyopathy, elevated diastolic wall strains trigger mechanotransduction pathways that initiate the addition of sarcomeres in series and an overall increase in myocyte length. At the whole organ level, this results in a chronic dilation of the ventricles, an increase in end diastolic and end systolic volumes, and a decrease in ejection fraction. However, how exactly changes in sarcomere number translate into changes in myocyte morphology, and how these cellular changes translate into ventricular dilation remains incompletely understood. Here we combined a chronic animal study, continuum growth modeling, and machine learning to quantify correlations between sarcomere dynamics, myocyte morphology, and ventricular dilation. In an eight-week long volume overload study of six pigs, we found that the average sarcomere number increased by +3.8%/week, from 47 to 62, resulting in a myocyte lengthening of +3.3%/week, from 85 to 108 μm, while the sarcomere length and myocyte width remained unchanged. At the same time, the average end diastolic volume increased by +6.0%/week. Using continuum growth modeling and Bayesian inference, we correlated alterations on the subcellular, cellular, and organ scales and found that the serial sarcomere number explained 88% of myocyte lengthening, which, in turn, explained 54% of cardiac dilation. Our results demonstrate that sarcomere number and myocyte length are closely correlated and constitute the major determinants of dilated heart failure. We anticipate our study to be a starting point for more sophisticated multiscale models of heart failure. Our study suggests that altering sarcomere turnover-and with it myocyte morphology and ventricular dimensions-could be a potential therapeutic target to attenuate or reverse the progression of heart failure. STATEMENT OF SIGNIFICANCE: Heart failure is a significant global health problem that affects more than 25 million people worldwide and increases in prevalence as the population ages. Heart failure has been studied excessively at various scales; yet, there is no compelling concept to connect knowledge from the subcellular, cellular, and organ level across the scales. Here we combined a chronic animal study, continuum growth modeling, and machine learning to quantify correlations between sarcomere dynamics, myocyte morphology, and ventricular dilation. We found that the serial sarcomere number explained 88% of myocyte lengthening, which, in turn, explained 54% of cardiac dilation. Our results show that sarcomere number and myocyte length are closely correlated and constitute the major determinants of dilated heart failure. This suggests that altering the sarcomere turnover-and with it myocyte morphology and ventricular dimensions-could be a potential therapeutic target to attenuate or reverse heart failure.
Keywords: Bayesian inference; Growth and remodeling; Heart failure; Machine learning; Multiscale modeling; Myocyte; Sarcomere; Uncertainty quantification.
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures








References
-
- Benjamin EJ, Salim SV, Clifton WC, Chang AR, Cheng S, et al. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 137 (2018) e67–e492 - PubMed
-
- Abilez OJ, Tzatzalos E, Yang H, Zhao MT, Jung G, Zöllner AM, Tiburcy M, Riegler J, Matsa E, Shukla P, Zhuge Y, Chour T, Chen VC, Burridge PW, Karakides I, Kuhl E, Bernstein D, Couture LA, Gold JD, Zimmermann WH, Wu JC. Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells. 36 (2018) 265–277 - PMC - PubMed
-
- Chabiniok R, Wang V, Hadjicharalambous M, Asner L, Lee J, Sermesant M, Kuhl E, Young A, Moireau P, Nash M, Chapelle D, Nordsletten DA. Multiphysics and multiscale modeling, data-model fusion and integration of organ physiology in the clinic: ventricular cardiac mechanics. Interface Focus 6 (2016) 20150083. - PMC - PubMed
-
- Chen YF, Said S, Campbell SE, Gerdes AM. A method to collect isolated myocytes and whole tissue from the same heart. Am. J. Physiol. Heart Circ. Physiol 293 (2007) 2004–2006 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

