Integrative Omics Reveals Metabolic and Transcriptomic Alteration of Nonalcoholic Fatty Liver Disease in Catalase Knockout Mice
- PMID: 30630288
- PMCID: PMC6430223
- DOI: 10.4062/biomolther.2018.175
Integrative Omics Reveals Metabolic and Transcriptomic Alteration of Nonalcoholic Fatty Liver Disease in Catalase Knockout Mice
Abstract
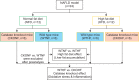
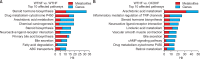
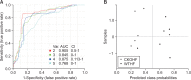
The prevalence of nonalcoholic fatty liver disease (NAFLD) has increased with the incidence of obesity; however, the underlying mechanisms are unknown. In this study, high-resolution metabolomics (HRM) along with transcriptomics were applied on animal models to draw a mechanistic insight of NAFLD. Wild type (WT) and catalase knockout (CKO) mice were fed with normal fat diet (NFD) or high fat diet (HFD) to identify the changes in metabolic and transcriptomic profiles caused by catalase gene deletion in correspondence with HFD. Integrated omics analysis revealed that cholic acid and 3β, 7α-dihydroxy-5-cholestenoate along with cyp7b1 gene involved in primary bile acid biosynthesis were strongly affected by HFD. The analysis also showed that CKO significantly changed all-trans-5,6-epoxy-retinoic acid or all-trans-4-hydroxy-retinoic acid and all-trans-4-oxo-retinoic acid along with cyp3a41b gene in retinol metabolism, and α/γ-linolenic acid, eicosapentaenoic acid and thromboxane A2 along with ptgs1 and tbxas1 genes in linolenic acid metabolism. Our results suggest that dysregulated primary bile acid biosynthesis may contribute to liver steatohepatitis, while up-regulated retinol metabolism and linolenic acid metabolism may have contributed to oxidative stress and inflammatory phenomena in our NAFLD model created using CKO mice fed with HFD.
Keywords: Catalase; Inflammation; Liver metabolism; Metabolomics; Nonalcoholic fatty liver disease; Transcriptomics.
Conflict of interest statement
The authors report no conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources

