Long-term results of glaucoma drainage device surgery
- PMID: 30630462
- PMCID: PMC6327392
- DOI: 10.1186/s12886-019-1027-z
Long-term results of glaucoma drainage device surgery
Abstract
Background: To evaluate long-term results of eyes with glaucoma drainage device (GDD).
Methods: We retrospectively reviewed medical records of all patients who underwent GDD placement at our institution between 2001 and 2014. A total of 110 eyes of 90 patients were studied. Glaucoma outcome was assessed by postoperative intraocular pressure (IOP), number of medications, and need for further glaucoma surgery. Surgical procedures before and during the study period, and their complications were evaluated.
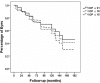
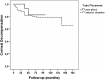
Results: The mean follow-up was 78.3 ± 44.0 months. The mean preoperative intraocular pressure was 30.8 ± 6.9 mmHg with 3.5 ± 1.1 glaucoma medications. At last postoperative follow-up, the mean IOP decreased to 14.3 ± 5.4 mmHg with 1.6 ± 1.5 glaucoma medications. GDD implantation successfully controlled glaucoma in 86, 85, 81, 78, 79, 76 and 73% of eyes at 1, 2, 3, 4, 5, 7 and 10 years, respectively. At last follow-up IOP was successfully controlled in 67% of eyes. Clinical complications occurred in 56.4% of eyes during the follow-up period.
Conclusions: A glaucoma drainage device can successfully control intractable glaucoma even after a very long period of time.
Keywords: Ahmed valve; Baerveldt implant; Glaucoma drainage device; Glaucoma surgery; IOP control.
Conflict of interest statement
Ethics approval and consent to participate
This study adhered to the tenets of the Declaration of Helsinki. For this retrospective type of study formal consent is not required according the regulations of the IRB Universitiy Medical Center Schleswig-Holstein.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical