Effectiveness of a decision aid for promoting colorectal cancer screening in Spain: a randomized trial
- PMID: 30630487
- PMCID: PMC6327535
- DOI: 10.1186/s12911-019-0739-6
Effectiveness of a decision aid for promoting colorectal cancer screening in Spain: a randomized trial
Abstract
Background: Colorectal cancer (CRC) screening has shown to reduce incidence and mortality rates, and therefore is widely recommended for people above 50 years-old. However, despite the implementation of population-based screening programs in several countries, uptake rates are still low. Decision aids (DAs) may help patients to make informed decisions about CRC screening.
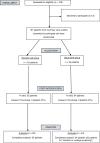
Methods: We performed a randomized controlled trial to assess the effectiveness of a DA developed to promote CRC screening, with patients from two primary care centers in Spain who never had underwent CRC screening. Contrary to center B (n = 24), Center A (n = 83) attended patients from an area where the population-based screening program was not implemented at that moment. Outcome measures were decisional conflict, knowledge of the disease and available screening options, intention to uptake the test, and concordance between patients' goals/concerns and intention.
Results: In center A, there were significant differences favoring the DA in decisional conflict (p < 0.001) and knowledge (p < 0.001). The absolute differences favoring DA group in intention to undergo fecal occult blood test (10.5%) and colonoscopy (13.7%) were significant only before correction for attenuation. In center B the differences were significant only for knowledge (p < 0.001). Patients' goals and concerns regarding the screening did not significantly predict their intention, and therefore we could not calculate a measure of concordance between the two constructs.
Conclusions: A DA improved the decisional process of participants who had never been invited to participate in the Spanish public CRC screening program, replicating previous results in this field. Future research is needed to identify subgroups that could benefit more from these interventions.
Trial registration: International Standard Registered Clinical/social Study Number: ISRCTN98108615 (Retrospectively registered on 27 December 2018).
Keywords: Colorectal cancer screening; Decision aid; Patient involvement; Primary care; Randomized controlled trial; Shared decision-making; Spain.
Conflict of interest statement
Ethics approval and consent to participate
The Scientific and Ethics Committee of the University Hospital Nuestra Señora de la Candelaria (Tenerife, Spain) approved the study protocol (file number: 2013/21). The study was performed in accordance with Good Clinical Practice standards, applicable local regulatory requirements and the recommendations of the Declaration of Helsinki.
This was a web-based study and the informed consent was built into the intervention. It was included in the intervention slides just after the welcome. It was an electronic consent document where the participant was required to accept to proceed with the study. If he/she did not consent the system thanked them for their interest but did not let them proceed. Control participants signed informed consent in a different interface of the web site.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- GLOBOCAN . Colorectal Cancer: Estimated incidence, mortality and prevalence worldwide in 2012. International Agency for Research on Cancer. 2012.
-
- Browse the SEER Cancer Statistics Review (CSR) 1975-2013. https://seer.cancer.gov/archive/csr/1975_2013/browse_csr.php?sectionSEL=.... Accessed 13 Jun 2017.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical