Two Distinct Sets of Ca2+ and K+ Channels Are Activated at Different Membrane Potentials by the Climbing Fiber Synaptic Potential in Purkinje Neuron Dendrites
- PMID: 30630881
- PMCID: PMC6507091
- DOI: 10.1523/JNEUROSCI.2155-18.2018
Two Distinct Sets of Ca2+ and K+ Channels Are Activated at Different Membrane Potentials by the Climbing Fiber Synaptic Potential in Purkinje Neuron Dendrites
Abstract
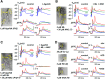
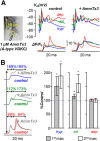
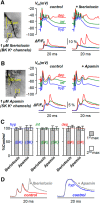
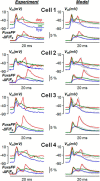
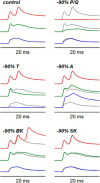
In cerebellar Purkinje neuron dendrites, the transient depolarization associated with a climbing fiber (CF) EPSP activates voltage-gated Ca2+ channels (VGCCs), voltage-gated K+ channels (VGKCs), and Ca2+-activated SK and BK K+ channels. The resulting membrane potential (Vm) and Ca2+ transients play a fundamental role in dendritic integration and synaptic plasticity of parallel fiber inputs. Here we report a detailed investigation of the kinetics of dendritic Ca2+ and K+ channels activated by CF-EPSPs, based on optical measurements of Vm and Ca2+ transients and on a single-compartment NEURON model reproducing experimental data. We first measured Vm and Ca2+ transients associated with CF-EPSPs at different initial Vm, and we analyzed the changes in the Ca2+ transients produced by the block of each individual VGCCs, of A-type VGKCs and of SK and BK channels. Then, we constructed a model that includes six active ion channels to accurately match experimental signals and extract the physiological kinetics of each channel. We found that two different sets of channels are selectively activated. When the dendrite is hyperpolarized, CF-EPSPs mainly activate T-type VGCCs, SK channels, and A-type VGKCs that limit the transient Vm ∼ <0 mV. In contrast, when the dendrite is depolarized, T-type VGCCs and A-type VGKCs are inactivated and CF-EPSPs activate P/Q-type VGCCs, high-voltage activated VGKCs, and BK channels, leading to Ca2+ spikes. Thus, the potentially activity-dependent regulation of A-type VGKCs, controlling the activation of this second set of channels, is likely to play a crucial role in signal integration and plasticity in Purkinje neuron dendrites.SIGNIFICANCE STATEMENT The climbing fiber synaptic input transiently depolarizes the dendrite of cerebellar Purkinje neurons generating a signal that plays a fundamental role in dendritic integration. This signal is mediated by two types of Ca2+ channels and four types of K+ channels. Thus, understanding the kinetics of all of these channels is crucial for understanding PN function. To obtain this information, we used an innovative strategy that merges ultrafast optical membrane potential and Ca2+ measurements, pharmacological analysis, and computational modeling. We found that, according to the initial membrane potential, the climbing fiber depolarizing transient activates two distinct sets of channels. Moreover, A-type K+ channels limit the activation of P/Q-type Ca2+ channels and associated K+ channels, thus preventing the generation of Ca2+ spikes.
Keywords: calcium channels; cerebellar Purkinje neuron; climbing fiber; neuron modeling; neuronal dendrites; potassium channels.
Copyright © 2019 the authors 0270-6474/19/391969-13$15.00/0.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
