Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes
- PMID: 30630901
- PMCID: PMC6413693
- DOI: 10.1126/science.aao5213
Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes
Abstract
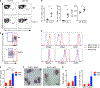
Cytopenias are an important clinical problem associated with inflammatory disease and infection. We show that specialized phagocytes that internalize red blood cells develop in Toll-like receptor 7 (TLR7)-driven inflammation. TLR7 signaling caused the development of inflammatory hemophagocytes (iHPCs), which resemble splenic red pulp macrophages but are a distinct population derived from Ly6Chi monocytes. iHPCs were responsible for anemia and thrombocytopenia in TLR7-overexpressing mice, which have a macrophage activation syndrome (MAS)-like disease. Interferon regulatory factor 5 (IRF5), associated with MAS, participated in TLR7-driven iHPC differentiation. We also found iHPCs during experimental malarial anemia, in which they required endosomal TLR and MyD88 signaling for differentiation. Our findings uncover a mechanism by which TLR7 and TLR9 specify monocyte fate and identify a specialized population of phagocytes responsible for anemia and thrombocytopenia associated with inflammation and infection.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
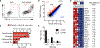
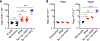

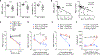


References
-
- Ravelli A, Grom AA, Behrens EM, Cron RQ, Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun 13, 289–298 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

