Venezuelan Equine Encephalitis Virus Capsid Implicated in Infection-Induced Cell Cycle Delay in vitro
- PMID: 30631316
- PMCID: PMC6315117
- DOI: 10.3389/fmicb.2018.03126
Venezuelan Equine Encephalitis Virus Capsid Implicated in Infection-Induced Cell Cycle Delay in vitro
Abstract
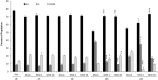
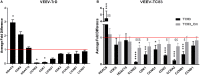
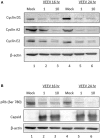
Venezuelan equine encephalitis virus (VEEV) is a positive sense, single-stranded RNA virus and member of the New World alphaviruses. It causes a biphasic febrile illness that can be accompanied by central nervous system involvement and moderate morbidity in humans and severe mortality in equines. The virus has a history of weaponization, lacks FDA-approved therapeutics and vaccines in humans, and is considered a select agent. Like other RNA viruses, VEEV replicates in the cytoplasm of infected cells and eventually induces apoptosis. The capsid protein, which contains a nuclear localization and a nuclear export sequence, induces a shutdown of host transcription and nucleocytoplasmic trafficking. Here we show that infection with VEEV causes a dysregulation of cell cycling and a delay in the G0/G1 phase in Vero cells and U87MG astrocytes. Cells infected with VEEV encoding a capsid NLS mutant or treated with the capsid-importin α interaction inhibitor G281-1485 were partially rescued from this cell cycle dysregulation. Pathway analysis of previously published RNA-sequencing data from VEEV infected U87MG astrocytes identified alterations of canonical pathways involving cell cycle, checkpoint regulation, and proliferation. Multiple cyclins including cyclin D1, cyclin A2 and cyclin E2 and other regulators of the cell cycle were downregulated in infected cells in a capsid NLS dependent manner. Loss of Rb phosphorylation, which is a substrate for cyclin/cdk complexes was also observed. These data demonstrate the importance of capsid nuclear localization and/or importin α binding for inducing cell cycle arrest and transcriptional downregulation of key cell cycle regulators.
Keywords: Venezuelan equine encephalitis virus; alphavirus; capsid; cell cycle; cyclin.
Figures
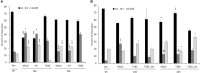
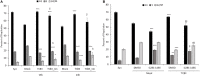
References
LinkOut - more resources
Full Text Sources
Research Materials

