Design of Peptide-Based Nanovaccines Targeting Leading Antigens From Gynecological Cancers to Induce HLA-A2.1 Restricted CD8+ T Cell Responses
- PMID: 30631324
- PMCID: PMC6315164
- DOI: 10.3389/fimmu.2018.02968
Design of Peptide-Based Nanovaccines Targeting Leading Antigens From Gynecological Cancers to Induce HLA-A2.1 Restricted CD8+ T Cell Responses
Abstract
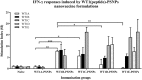
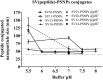
Gynecological cancers are a leading cause of mortality in women. CD8+ T cell immunity largely correlates with enhanced survival, whereas inflammation is associated with poor prognosis. Previous studies have shown polystyrene nanoparticles (PSNPs) are biocompatible, do not induce inflammation and when used as vaccine carriers for model peptides induce CD8+ T cell responses. Herein we test the immunogenicity of 24 different peptides, from three leading vaccine target proteins in gynecological cancers: the E7 protein of human papilloma virus (HPV); Wilms Tumor antigen 1 (WT1) and survivin (SV), in PSNP conjugate vaccines. Of relevance to vaccine development was the finding that a minimal CD8+ T cell peptide epitope from HPV was not able to induce HLA-A2.1 specific CD8+ T cell responses in transgenic humanized mice using conventional adjuvants such as CpG, but was nevertheless able to generate strong immunity when delivered as part of a specific longer peptide conjugated to PSNPs vaccines. Conversely, in most cases, when the minimal CD8+ T cell epitopes were able to induce immune responses (with WT1 or SV super agonists) in CpG, they also induced responses when conjugated to PSNPs. In this case, extending the sequence around the CD8+ T cell epitope, using the natural protein context, or engineering linker sequences proposed to enhance antigen processing, had minimal effects in enhancing or changing the cross-reactivity pattern induced by the super agonists. Nanoparticle approaches, such as PSNPs, therefore may offer an alternative vaccination strategy when conventional adjuvants are unable to elicit the desired CD8+ T cell specificity. The findings herein also offer sequence specific insights into peptide vaccine design for nanoparticle-based vaccine carriers.
Keywords: CD8 T cell epitopes; HLA-A2.1; HPV; WT1; immunogenicity; nanoparticles; survivin; vaccine.
Figures





References
-
- Australia AIHW. Gynaecological Cancers in Australia: an Overview (Canberra, ACT: ) (2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

