Inflammatory cell expression of Toll-like receptor-2 (TLR2) within refractory periapical granuloma
- PMID: 30631444
- PMCID: PMC6281009
- DOI: 10.12688/f1000research.16678.1
Inflammatory cell expression of Toll-like receptor-2 (TLR2) within refractory periapical granuloma
Abstract
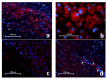
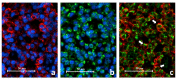
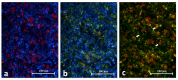
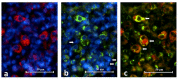
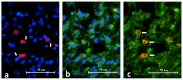
Background: Toll-like receptor-2 (TLR2) is highly important within the immune system. Characterization of the expression of TLR2 within inflammatory cells in periapical lesions could help in diagnosis and management of refractory cases. The aim of the study is identification of Toll-like receptor (TLR2) through immunohistochemical and immunofluroscence expression in inflammatory cells within refractory periapical granuloma cases. Methods: Eight cases of refractory periapical granuloma were selected out of 772 cases. Histological examination and immunohistochemical staining with polyclonal rabbit antihuman TLR2, monoclonal mouse antihuman CD38, CD68 and CD83 primary antibodies, as well as immunofluorescence staining with goat anti-rabbit TLR2, donkey anti-mouse CD38, CD68 and CD83 primary antibodies was conducted. Positive controls, negative controls and experimental sections with no primary antibody were included in the study. Qualitative analysis and double immunofluorescence technique was used to characterize the TLR + cells. Results: In periapical granuloma, lymphocytes (CD38 cells) expressed the most amount of TLR reactivity followed by macrophages (CD68 cells), and odontogenic epithelial cells. Neutrophils, red blood cells (RBCs) and collagen ground substance were negative to TLR2. Conclusion: TLR2 was highly expressed by lymphocytes and plasma cells indicative of their major role in the inflammatory process and antigen recognition in refractory periapical granuloma. Dendritic cells expressing TLR2 were low in number suggesting a minor role in sustaining these lesions.
Keywords: Antigen presenting cells; chronic inflammatory cells; immune response; periapical granuloma; toll-like receptors..
Conflict of interest statement
No competing interests were disclosed.
Figures








References
-
- Sundqvist G, Figdor D: Life as an endodontic pathogen. Endodontic Topics. 2003;6(1):3–28. 10.1111/j.1601-1546.2003.00054.x - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

