Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up
- PMID: 30632023
- PMCID: PMC6438948
- DOI: 10.1007/s10549-018-05125-4
Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up
Abstract
Purpose: In the initial PALOMA-2 (NCT01740427) analysis with median follow-up of 23 months, palbociclib plus letrozole significantly prolonged progression-free survival (PFS) in women with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC) [hazard ratio (HR) 0.58; P < 0.001]. Herein, we report results overall and by subgroups with extended follow-up.
Methods: In this double-blind, phase 3 study, post-menopausal women with ER+/HER2- ABC who had not received prior systemic therapy for their advanced disease were randomized 2:1 to palbociclib-letrozole or placebo-letrozole. Endpoints include investigator-assessed PFS (primary), safety, and patient-reported outcomes (PROs).
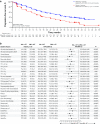
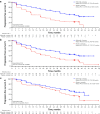
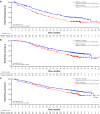
Results: After a median follow-up of approximately 38 months, median PFS was 27.6 months for palbociclib-letrozole (n = 444) and 14.5 months for placebo-letrozole (n = 222) (HR 0.563; 1-sided P < 0.0001). All subgroups benefited from palbociclib treatment. The improvement of PFS with palbociclib-letrozole was maintained in the next 2 subsequent lines of therapy and delayed the use of chemotherapy (40.4 vs. 29.9 months for palbociclib-letrozole vs. placebo-letrozole). Safety data were consistent with the known profile. Patients' quality of life was maintained.
Conclusions: With approximately 15 months of additional follow-up, palbociclib plus letrozole continued to demonstrate improved PFS compared with placebo plus letrozole in the overall population and across all patient subgroups, while the safety profile remained favorable and quality of life was maintained. These data confirm that palbociclib-letrozole should be considered the standard of care for first-line therapy in patients with ER+/HER2- ABC, including those with low disease burden or long disease-free interval. Sponsored by Pfizer; ClinicalTrials.gov: NCT01740427.
Keywords: Breast cancer; Cyclin-dependent kinase inhibitor; ER+; HER2−; Letrozole; Palbociclib.
Conflict of interest statement
Conflict of interest
HSR’s institution received research funding from Plexxikon, Macrogenics, OBI Pharma, Eisai, Pfizer, Novartis, Eli Lilly, Roche, and Merck. RSF received consulting fees from Pfizer, Bayer, Novartis, Bristol-Myers Squibb, Merck, Eli Lilly, Eisai, and Roche/Genentech as well as other research funding from Pfizer. VD received consulting fees from Genentech, Eli Lilly, Pfizer, Astellas, AbbVie, Novartis Pharma KK, Daichy, Tesaro, NSD, Seattle Genetics, and Roche-Peru, as well as speakers bureau fees from Pfizer, Novartis Pharma KK, and Roche-Peru. JE received consulting fees from Eli Lilly, Novartis, Pfizer, Roche, and Eisai, performed contracted research for Celgene, and received honoraria from Eli Lilly, Novartis, Pfizer, Roche, TEVA, and Pierre Fabre. AAJ received consulting fees from Amgen, AbbVie, AstraZeneca, BMS, Eli Lilly, Pfizer, Novartis, and Roche. NH received consulting fees for Eli Lilly, Novartis, and Pfizer. AC received consulting/advisory fees from Agendia. KAG received consulting/advisory fees from Pfizer, Novartis, AstraZeneca, NanoString Technologies, Merck, Eli Lilly, Genomic Health, Roche, Seattle Genetics, and Mylan. DJS received consulting fees from Pfizer, Eli Lilly, and Novartis, performed contracted research for Pfizer and Novartis, is a Pfizer stockholder, received travel accommodation/expenses from Pfizer and BioMarin, and has a leadership role with BioMarin. CHB, ERG, SI, DRL, and AM are employees of and own stock in Pfizer. OL has nothing to disclose.
Ethical approval
This trial complies with the current laws of the countries in which it was performed.
Figures

References
-
- Cardoso F, Costa A, Senkus E, Aapro M, Andre F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso MJ, Carey L, Corneliussen-James D, Curigliano G, Dieras V, El Saghir N, Eniu A, Fallowfield L, Fenech D, Francis P, Gelmon K, Gennari A, Harbeck N, Hudis C, Kaufman B, Krop I, Mayer M, Meijer H, Mertz S, Ohno S, Pagani O, Papadopoulos E, Peccatori F, Pernault-Llorca F, Piccart MJ, Pierga JY, Rugo H, Shockney L, Sledge G, Swain S, Thomssen C, Tutt A, Vorobiof D, Xu B, Norton L, Winer E. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3) Breast. 2017;31:244–259. doi: 10.1016/j.breast.2016.10.001. - DOI - PubMed
-
- Rugo H, Rumble B, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield LA, Fowble B, Ingle JN, Jahanzeb M, Johnston SR, Korde LA, Khatcheressian J, M RS, Muss HB, Burstein HJ. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. - DOI - PubMed
-
- National Comprehensive Cancer Network (2017) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast cancer. Version 4.2017 https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed Feb 12 2018 - PubMed
-
- IBRANCE® (palbociclib) Full prescribing information. New York: Pfizer Inc; 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

