A phase 1b randomized, placebo-controlled clinical trial with SNF472 in haemodialysis patients
- PMID: 30632182
- PMCID: PMC6422667
- DOI: 10.1111/bcp.13863
A phase 1b randomized, placebo-controlled clinical trial with SNF472 in haemodialysis patients
Abstract
Aims: SNF472 is a calcification inhibitor that is being studied as a novel treatment for calciphylaxis and cardiovascular calcification (CVC). A first study showed acceptable safety and tolerability in a single ascending dose administration in healthy volunteers and a single dose administration in haemodialysis (HD) patients. This study aimed to assess the safety, tolerability, and pharmacokinetics/pharmacodynamics relationship of intravenous SNF472 in HD patients in a multiple ascending dose administration trial with 5 doses tested for 1 week (3 administrations) and 1 dose tested for 4 weeks (12 administrations).
Methods: This double blind, randomized, placebo-controlled Phase 1b study investigated the safety, tolerability, pharmacokinetics and pharmacodynamics of SNF472 after repeated administrations to HD patients for up to 28 days. A pharmacodynamic assessment was performed to evaluate the potential for SNF472 to inhibit hydroxyapatite (HAP) formation. Patients were grouped into 2 cohorts, receiving multiple ascending doses for 1 week (1 to 20 mg/kg, Cohort 1) and 1 dose of 10 mg/kg for 4 weeks (Cohort 2) of intravenous SNF472.
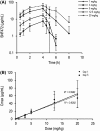
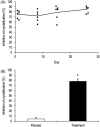
Results: Physical status, body weight, cardiorespiratory function, body temperature and laboratory parameters were in the normal range. No clinically relevant effects on heart rate or blood pressure were observed. No abnormal electrocardiogram or QTcB period were reported. The peak plasma concentration (7.6, 16.1, 46.0 and 66.9 μg/mL for 3, 5, 12.5 and 20 mg/kg, respectively) was observed at the end of the 4-hour infusion and thereafter concentrations declined rapidly with half-life between 32 and 65 min. SNF472 at 10 mg/kg inhibited dose dependently HAP crystallization in plasma samples after 28 days of treatment (78% inhibition, P < .001).
Conclusions: SNF472 is safe and well tolerated in HD patients after 2 schemes: multiple ascending doses for 1 week and after repeated dosing of 10 mg/kg for 4 weeks. In both schemes, SNF472 inhibits the induction of HAP crystallization. These results provide support for the use of SNF472 as a novel treatment for CVC in end-stage renal disease.
Keywords: SNF472; cardiovascular calcification; haemodialysis; hydroxyapatite; myo-inositol hexaphosphate; renal disease.
Conflict of interest statement
J.P., P.H.J., M.D.F., A.Z.C. and C.S. are employees or have received honoraria from Laboratoris Sanifit SL and are shareholders at Laboratoris Sanifit SL.
Figures

References
-
- Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end‐stage renal disease. Hypertension. 2001;38(4):938‐942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

