Chronic treatment with N-acetylcysteine decreases extinction responding and reduces cue-induced nicotine-seeking
- PMID: 30632301
- PMCID: PMC6328917
- DOI: 10.14814/phy2.13958
Chronic treatment with N-acetylcysteine decreases extinction responding and reduces cue-induced nicotine-seeking
Abstract
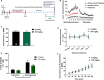
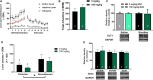
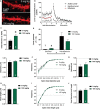
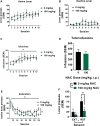
N-acetylcysteine (NAC), a promising glutamatergic therapeutic agent, has shown some clinical efficacy in reducing nicotine use in humans and has been shown to reverse drug-induced changes in glutamatergic neurophysiology. In rats, nicotine-seeking behavior is associated with alterations in glutamatergic plasticity within the nucleus accumbens core (NAcore). Specifically, cue-induced nicotine-seeking is associated with rapid, transient synaptic plasticity (t-SP) in glutamatergic synapses on NAcore medium spiny neurons. The goal of the present study was to determine if NAC reduces nicotine-seeking behavior and reverses reinstatement-associated NAcore glutamatergic alterations. Rats were extinguished from nicotine self-administration, followed by subchronic NAC administration (0 or 100 mg/kg/d) for 4 days prior to cue-induced reinstatement. NAcore synaptic potentiation was measured via dendritic spine morphology and mRNA and protein of relevant glutamatergic genes were quantified. Nicotine-seeking behavior was not reduced by subchronic NAC treatment. Also, NAcore transcript and protein expression of multiple glutamatergic genes, as well as spine morphological measures, were unaffected by subchronic NAC. Finally, chronic NAC treatment (15 days total) during extinction and prior to reinstatement significantly decreased extinction responding and reduced reinstatement of nicotine-seeking compared to vehicle. Together, these results suggest that chronic NAC treatment is necessary for its therapeutic efficacy as a treatment strategy for nicotine addiction and relapse.
Keywords: N-acetylcysteine; Nicotine; Relapse; Synaptic Plasticity.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures


References
-
- Ahmed, S. H. , and Koob G. F.. 1998. Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300. - PubMed
-
- Ahmed, S. H. , Walker J. R., and Koob G. F.. 2000. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

