Ultrathin Dual-Scale Nano- and Microporous Membranes for Vascular Transmigration Models
- PMID: 30632319
- PMCID: PMC6530565
- DOI: 10.1002/smll.201804111
Ultrathin Dual-Scale Nano- and Microporous Membranes for Vascular Transmigration Models
Abstract
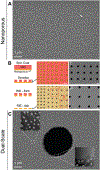
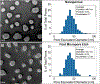
Selective cellular transmigration across the microvascular endothelium regulates innate and adaptive immune responses, stem cell localization, and cancer cell metastasis. Integration of traditional microporous membranes into microfluidic vascular models permits the rapid assay of transmigration events but suffers from poor reproduction of the cell permeable basement membrane. Current microporous membranes in these systems have large nonporous regions between micropores that inhibit cell communication and nutrient exchange on the basolateral surface reducing their physiological relevance. Here, the use of 100 nm thick continuously nanoporous silicon nitride membranes as a base substrate for lithographic fabrication of 3 µm pores is presented, resulting in a highly porous (≈30%), dual-scale nano- and microporous membrane for use in an improved vascular transmigration model. Ultrathin membranes are patterned using a precision laser writer for cost-effective, rapid micropore design iterations. The optically transparent dual-scale membranes enable complete observation of leukocyte egress across a variety of pore densities. A maximal density of ≈14 micropores per cell is discovered beyond which cell-substrate interactions are compromised giving rise to endothelial cell losses under flow. Addition of a subluminal extracellular matrix rescues cell adhesion, allowing for the creation of shear-primed endothelial barrier models on nearly 30% continuously porous substrates.
Keywords: dual-scale; nanoporous silicon nitride; shear stress; transmigration; vascular mimetic.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of Interest
The authors declare the following competing financial interest(s): JLM and TRG are co-founders of SiMPore, an early-stage company commercializing ultrathin silicon-based technologies.
Figures
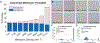




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

