A Combination of Tri-Leucine and Angiopep-2 Drives a Polyanionic Polymalic Acid Nanodrug Platform Across the Blood-Brain Barrier
- PMID: 30633492
- PMCID: PMC7641102
- DOI: 10.1021/acsnano.8b06437
A Combination of Tri-Leucine and Angiopep-2 Drives a Polyanionic Polymalic Acid Nanodrug Platform Across the Blood-Brain Barrier
Abstract
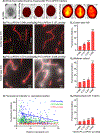
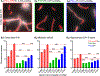
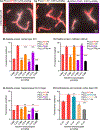
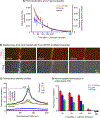
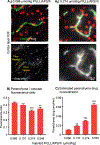
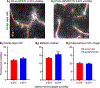
One of the major problems facing the treatment of neurological disorders is the poor delivery of therapeutic agents into the brain. Our goal is to develop a multifunctional and biodegradable nanodrug delivery system that crosses the blood-brain barrier (BBB) to access brain tissues affected by neurological disease. In this study, we synthesized a biodegradable nontoxic β-poly(l-malic acid) (PMLA or P) as a scaffold to chemically bind the BBB crossing peptides Angiopep-2 (AP2), MiniAp-4 (M4), and the transferrin receptor ligands cTfRL and B6. In addition, a trileucine endosome escape unit (LLL) and a fluorescent marker (rhodamine or rh) were attached to the PMLA backbone. The pharmacokinetics, BBB penetration, and biodistribution of nanoconjugates were studied in different brain regions and at multiple time points via optical imaging. The optimal nanoconjugate, P/LLL/AP2/rh, produced significant fluorescence in the parenchyma of cortical layers II/III, the midbrain colliculi, and the hippocampal CA1-3 cellular layers 30 min after a single intravenous injection; clearance was observed after 4 h. The nanoconjugate variant P/LLL/rh lacking AP2, or the variant P/AP2/rh lacking LLL, showed significantly less BBB penetration. The LLL moiety appeared to stabilize the nanoconjugate, while AP2 enhanced BBB penetration. Finally, nanoconjugates containing the peptides M4, cTfRL, and B6 displayed comparably little and/or inconsistent infiltration of brain parenchyma, likely due to reduced trans-BBB movement. P/LLL/AP2/rh can now be functionalized with intra-brain targeting and drug treatment moieties that are aimed at molecular pathways implicated in neurological disorders.
Keywords: Angiopep-2; blood−brain barrier; optical analysis; polymalic acid; targeting peptide; treatment of neurological disorder.
Figures


References
-
- Dréan A; Goldwirt L; Verreault M; Canney M; Schmitt C; Guehennec J; Delattre J-Y; Carpentier A; Idbaih A Blood-Brain Barrier, Cytotoxic Chemotherapies and Glioblastoma. Expert Rev. Neurother. 2016, 16, 1285–1300. - PubMed
-
- Abbott NJ Blood–Brain Barrier Structure and Function and the Challenges for CNS Drug Delivery. J. Inherited Metab. Dis. 2013, 36, 437–449. - PubMed
-
- Lopes S; Ribeiro F; Wojnarowicz J; Ƚojkowski W; Jurkschat K; Crossley A; Soares AM; Loureiro S Zinc Oxide Nanoparticles Toxicity to Daphnia Magna: Size-Dependent Effects and Dissolution. Environ. Toxicol. Chem. 2014, 33, 190–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

