Inhaled nitric oxide to treat intermediate risk pulmonary embolism: A multicenter randomized controlled trial
- PMID: 30633959
- PMCID: PMC7047892
- DOI: 10.1016/j.niox.2019.01.006
Inhaled nitric oxide to treat intermediate risk pulmonary embolism: A multicenter randomized controlled trial
Abstract
Objective: To test the hypothesis that adjunctive inhaled NO would improve RV function and viability in acute PE.
Methods: This was a randomized, placebo-controlled, double blind trial conducted at four academic hospitals. Eligible patients had acute PE without systemic arterial hypotension but had RV dysfunction and a treatment plan of standard anticoagulation. Subjects received either oxygen plus 50 parts per million nitrogen (placebo) or oxygen plus 50 ppm NO for 24 h. The primary composite endpoint required a normal RV on echocardiography and a plasma troponin T concentration <14 pg/mL. The secondary endpoint required a blood brain natriuretic peptide concentration <90 pg/mL and a Borg dyspnea score ≤ 2. The sample size of N = 76 tested if 30% more patients treated with NO would achieve the primary endpoint with 80% power and alpha = 5%.
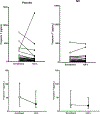
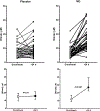
Results: We randomized 78 patients and after two withdrawals, 38 were treated per protocol in each group. Patients were well matched for baseline conditions. At 24 h, 5/38 (13%) of patients treated with placebo and 9/38 (24%) of patients treated with NO reached the primary endpoint (P = 0.375). The secondary endpoint was reached in 34% with placebo and 13% of the NO (P = 0.11). In a pre-planned post-hoc analysis, we examined how many patients with RV hypokinesis or dilation at enrollment resolved these abnormalities; 29% more patients treated with NO resolved both abnormalities at 24 h (P = 0.010, Cochrane's Q test).
Conclusions: In patients with severe submassive PE, inhaled nitric oxide failed to increase the proportion of patients with a normal troponin and echocardiogram but increased the probability of eliminating RV hypokinesis and dilation on echocardiography.
Clinical trial registration: NCT01939301.
Keywords: Brain natriuretic peptide; Echocardiography; Heart failure; Nitric oxide; Pulmonary embolism; Pulmonary hypertension; Randomized trial; Troponin.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- McIntyre KM, Sasahara AA: Determinants of right ventricular function and hemodynamics after pulmonary embolism. Chest 1974, 65(5):534–543. - PubMed
-
- Watts JA, Zagorski J, Gellar MA et al.: Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol 2006, 41(2):296–307. - PubMed
-
- Lankeit M, Jimenez D, Kostrubiec M et al.: Predictive value of the high-sensitivity troponin T assay and the simplified pulmonary embolism severity index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation 2011, 124(24):2716–2724. - PubMed
-
- Elliott CG: Pulmonary physiology during pulmonary embolism. Chest 1992, 101(4 Suppl):163s–171s. - PubMed
-
- Sanchez O, Trinquart L, Colombet I et al.: Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. EurHeart J 2008, 29(12):1569–1577. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

