Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance
- PMID: 30633973
- PMCID: PMC6377860
- DOI: 10.1016/j.ejpb.2019.01.006
Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance
Abstract
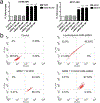
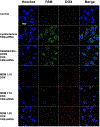
Multidrug resistance (MDR) significantly decreases the therapeutic efficiency of anti-cancer drugs. Its reversal could serve as a potential method to restore the chemotherapeutic efficiency. Downregulation of MDR-related proteins with a small interfering RNA (siRNA) is a promising way to reverse the MDR effect. Additionally, delivery of small molecule therapeutics simultaneously with siRNA can enhance the efficiency of chemotherapy by dual action in MDR cell lines. Here, we conjugated the dendrimer, generation 4 polyamidoamine (G4 PAMAM), with a polyethylene glycol (PEG)-phospholipid copolymer. The amphiphilic conjugates obtained spontaneously self-assembled into a micellar nano-preparation, which can be co-loaded with siRNA onto PAMAM moieties and sparingly water-soluble chemotherapeutics into the lipid hydrophobic core. This system was co-loaded with doxorubicin (DOX) and therapeutic siRNA (siMDR-1) and tested for cytotoxicity against MDR cancer cells: human ovarian carcinoma (A2780 ADR) and breast cancer (MCF7 ADR). The combination nanopreparation effectively downregulated P-gp in MDR cancer cells and reversed the resistance towards DOX.
Keywords: Chemotherapy; Combination delivery; Micelle; Mixed dendrimer micelles (MDM); Multidrug resistance; Polyamidoamine (PAMAM); siRNA delivery.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures


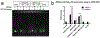
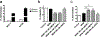
References
-
- Sarisozen C, et al. , Polymers in the co-delivery of siRNA and anticancer drugs to treat multidrug-resistant tumors. Journal of Pharmaceutical Investigation, 2017. 47(1): p. 37–49.
-
- Rodriguez-Antona C and Ingelman-Sundberg M, Cytochrome P450 pharmacogenetics and cancer. Oncogene, 2006. 25(11): p. 1679–91. - PubMed
-
- Shen H, et al. , Comparative metabolic capabilities and inhibitory profiles of CYP2D6.1, CYP2D6.10, and CYP2D6.17. Drug Metab Dispos, 2007. 35(8): p. 1292–300. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

