9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol
- PMID: 30634427
- PMCID: PMC6358742
- DOI: 10.3390/molecules24020220
9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol
Abstract
Lignans, neolignans, norlignans and norneolignans constitute a large class of phenolic natural compounds. 9-Norlignans, here defined to contain a β⁻β' bond between the two phenylpropanoid units and to lack carbon number 9 from the parent lignan structure, are the most rarely occurring compounds within this class of natural compounds. We present here an overview of the structure, occurrence and biological activity of thirty-five 9-norlignans reported in the literature to date. In addition, we report the semisynthetic preparation of sixteen 9-norlignans using the natural lignan hydroxymatairesinol obtained from spruce knots, as starting material. 9-Norlignans are shown to exist in different species and to have various biological activities, and they may therefore serve as lead compounds for example for the development of anticancer agents. Hydroxymatairesinol is shown to be a readily available starting material for the preparation of norlignans of the imperanene, vitrofolal and noralashinol family.
Keywords: 9-norlignans; bioactivity; hydroxymatairesinol; lignans; norlignans; semisynthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

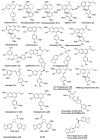

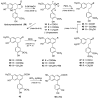
References
-
- Kawazoe K., Yutani A., Tamemoto K., Yuasa S., Shibata H., Higuti T., Takaishi Y. Phenylnaphthalene Compounds from the Subterranean Part of Vitex rotundifolia and Their Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus. J. Nat. Prod. 2001;64:588–591. doi: 10.1021/np000307b. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

