Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia
- PMID: 30634444
- PMCID: PMC6356970
- DOI: 10.3390/cancers11010060
Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia
Abstract
Regions of low oxygenation (hypoxia) are a characteristic feature of solid tumors, and cells existing in these regions are a major factor influencing radiation resistance as well as playing a significant role in malignant progression. Consequently, numerous pre-clinical and clinical attempts have been made to try and overcome this hypoxia. These approaches involve improving oxygen availability, radio-sensitizing or killing the hypoxic cells, or utilizing high LET (linear energy transfer) radiation leading to a lower OER (oxygen enhancement ratio). Interestingly, hyperthermia (heat treatments of 39⁻45 °C) induces many of these effects. Specifically, it increases blood flow thereby improving tissue oxygenation, radio-sensitizes via DNA repair inhibition, and can kill cells either directly or indirectly by causing vascular damage. Combining hyperthermia with low LET radiation can even result in anti-tumor effects equivalent to those seen with high LET. The various mechanisms depend on the time and sequence between radiation and hyperthermia, the heating temperature, and the time of heating. We will discuss the role these factors play in influencing the interaction between hyperthermia and radiation, and summarize the randomized clinical trials showing a benefit of such a combination as well as suggest the potential future clinical application of this combination.
Keywords: hyperthermia; hypoxia; radiation therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

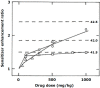
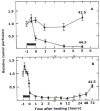

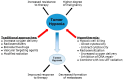
References
-
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989;49:6449–6465. - PubMed
-
- Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Res. 1986;46:467–473. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

