Specialized Roles for Actin in Osteoclasts: Unanswered Questions and Therapeutic Opportunities
- PMID: 30634501
- PMCID: PMC6359508
- DOI: 10.3390/biom9010017
Specialized Roles for Actin in Osteoclasts: Unanswered Questions and Therapeutic Opportunities
Abstract
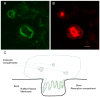
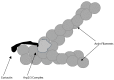
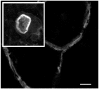
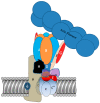
Osteoclasts are cells of the hematopoietic lineage that are specialized to resorb bone. In osteoclasts, the actin cytoskeleton engages in at least two unusual activities that are required for resorption. First, microfilaments form a dynamic and structurally elaborate actin ring. Second, microfilaments bind vacuolar H⁺-ATPase (V-ATPase) and are involved in forming the V-ATPase-rich ruffled plasma membrane. The current review examines these two specialized functions with emphasis on the identification of new therapeutic opportunities. The actin ring is composed of substructures called podosomes that are interwoven to form a cohesive superstructure. Studies examining the regulation of the formation of actin rings and its constituent proteins are reviewed. Areas where there are gaps in the knowledge are highlighted. Microfilaments directly interact with the V-ATPase through an actin binding site in the B2-subunit of V-ATPase. This binding interaction is required for ruffled membrane formation. Recent studies show that an inhibitor of the interaction blocks bone resorption in pre-clinical animal models, including a model of post-menopausal osteoporosis. Because the unusual actin-based resorption complex is unique to osteoclasts and essential for bone resorption, it is likely that deeper understanding of its underlying mechanisms will lead to new approaches to treat bone disease.
Keywords: V-ATPase; actin; actin polymerization; anti-resorptives; bone; bone remodeling; microfilament; vacuolar H+-ATPase.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Vaananen H.K., Karhukorpi E.K., Sundquist K., Wallmark B., Roininen I., Hentunen T., Tuukkanen J., Lakkakorpi P. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J. Cell Biol. 1990;111:1305–1311. doi: 10.1083/jcb.111.3.1305. - DOI - PMC - PubMed
-
- Bromme D., Okamoto K., Wang B.B., Biroc S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J. Biol. Chem. 1996;271:2126–2132. doi: 10.1074/jbc.271.4.2126. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

