Targeting Proteotoxic Stress in Cancer: A Review of the Role that Protein Quality Control Pathways Play in Oncogenesis
- PMID: 30634515
- PMCID: PMC6356294
- DOI: 10.3390/cancers11010066
Targeting Proteotoxic Stress in Cancer: A Review of the Role that Protein Quality Control Pathways Play in Oncogenesis
Abstract
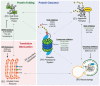
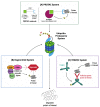
Despite significant advances in cancer diagnostics and therapeutics the majority of cancer unfortunately remains incurable, which has led to continued research to better understand its exceptionally diverse biology. As a result of genomic instability, cancer cells typically have elevated proteotoxic stress. Recent appreciation of this functional link between the two secondary hallmarks of cancer: aneuploidy (oxidative stress) and proteotoxic stress, has therefore led to the development of new anticancer therapies targeting this emerging "Achilles heel" of malignancy. This review highlights the importance of managing proteotoxic stress for cancer cell survival and provides an overview of the integral role proteostasis pathways play in the maintenance of protein homeostasis. We further review the efforts undertaken to exploit proteotoxic stress in multiple myeloma (as an example of a hematologic malignancy) and triple negative breast cancer (as an example of a solid tumor), and give examples of: (1) FDA-approved therapies in routine clinical use; and (2) promising therapies currently in clinical trials. Finally, we provide new insights gleaned from the use of emerging technologies to disrupt the protein secretory pathway and repurpose E3 ligases to achieve targeted protein degradation.
Keywords: autophagy; chemoresistance; multiple myeloma; proteasome; protein quality control; proteotoxic stress; triple negative breast cancer; unfolded protein response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

