Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds
- PMID: 30634523
- PMCID: PMC6359322
- DOI: 10.3390/s19020233
Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds
Abstract
Volatile organic compounds (VOCs), which originate from painting, oil refining and vehicle exhaust emissions, are hazardous gases that have significant effects on air quality and human health. The detection of VOCs is of special importance to environmental safety. Among the various detection methods, chemoresistive semiconductor metal oxide gas sensors are considered to be the most promising technique due to their easy production, low cost and good portability. Sensitivity is an important parameter of gas sensors and is greatly affected by the microstructure, defects, catalyst, heterojunction and humidity. By adjusting the aforementioned factors, the sensitivity of gas sensors can be improved further. In this review, attention will be focused on how to improve the sensitivity of chemoresistive gas sensors towards certain common VOCs with respect to the five factors mentioned above.
Keywords: gas sensor; metal oxide; semiconductor; sensitivity; volatile organic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



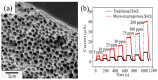


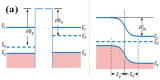

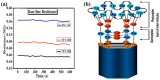





References
-
- Wang H.L., Nie L., Li J., Wang Y.F., Wang G., Wang J.H., Hao Z.P. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013;58:724–730.
-
- Ralf K. Volatile Organic Compounds in the Atmosphere. Blackwell; Oxford, UK: 2007. pp. 1–500.
-
- Ren F., Gao L., Yuan Y., Zhang Y., Alqrni A., Al-Dossary O.M., Xu J. Enhanced BTEX gas-sensing performance of CuO/SnO2 composite. Sens. Actuators B Chem. 2016;223:914–920.
-
- Yan H., Song P., Zhang S., Zhang J., Yang Z., Wang Q. Au nanoparticles modified MoO3 nanosheets with their enhanced properties for gas sensing. Sens. Actuators B Chem. 2016;236:201–207.
-
- Liu X., Iocozzia J., Wang Y., Cui X., Chen Y., Zhao S., Li Z., Lin Z. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017;10:402–434.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

