Electrochemical Detection for Uric Acid Based on β-Lactoglobulin-Functionalized Multiwall Carbon Nanotubes Synthesis with PtNPs Nanocomposite
- PMID: 30634585
- PMCID: PMC6356623
- DOI: 10.3390/ma12020214
Electrochemical Detection for Uric Acid Based on β-Lactoglobulin-Functionalized Multiwall Carbon Nanotubes Synthesis with PtNPs Nanocomposite
Abstract
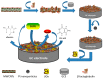
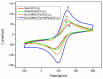
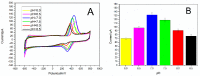
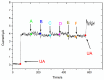
In this work, a simple and highly selective electrochemical biosensor for determination of uric acid (UA) is synthesized by using β-lactoglobulin (BLG)-functionalized multiwall carbon nanotubes (MWCNTs) and a platinum nanoparticles (PtNPs) nanocomposite. Urate oxidase (UOx) can oxidize uric acid to hydrogen peroxide and allantoin, which provides a good opportunity for electrochemical detection for UA. Under the optimized conditions, the current changes by the UOx/Bull Serum Albumin (BSA)/BLG-MWCNTs-PtNPs/Glassy Carbon (GC) electrode with the electrochemical method was proportional to the concentration of UA. According to experiments, we obtained a linear response with a concentration range from 0.02 to 0.5 mM and achieved a high sensitivity of 31.131 μA mM-1 and a low detection limit (0.8 μΜ). Meanwhile, nanoparticles improved the performance of the biosensor and combined with BLG not only prevented the accumulation of composite nanomaterials, but also provided immobilization of uricase through electrostatic adsorption. This improves the stability and gives the constructed electrode sensing interface superior performance in UA detection.
Keywords: multiwall carbon nanotubes; nanocomposite; urate oxidase; uric acid biosensor; β-lactoglobulin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sinha A., Lu X., Wu L., Tan D., Li Y., Chen J., Jain R. Voltammetric sensing of biomolecules at carbon based electrode interfaces: A review. Trac Trends Anal. Chem. 2018;98:174–189.
-
- Yang N., Chen X., Ren T., Zhang P., Yang D. Carbon nanotube based biosensors. Sens. Actuators B Chem. 2015;207:690–715. doi: 10.1016/j.snb.2014.10.040. - DOI
-
- Narang J., Sharma K.R., Chauhan N., Mishra A. Amplified electrochemical signal taking polyanline as sensing interface compared to polyindole carboxylic acid. Synth. Met. 2015;203:54–58. doi: 10.1016/j.synthmet.2015.02.019. - DOI
-
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56. doi: 10.1038/354056a0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

