Functional Assessment for Clinical Use of Serum-Free Adapted NK-92 Cells
- PMID: 30634595
- PMCID: PMC6356567
- DOI: 10.3390/cancers11010069
Functional Assessment for Clinical Use of Serum-Free Adapted NK-92 Cells
Abstract
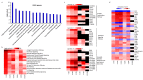
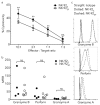
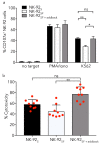
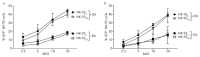
Natural killer (NK) cells stand out as promising candidates for cellular immunotherapy due to their capacity to kill malignant cells. However, the therapeutic use of NK cells is often dependent on cell expansion and activation with considerable amounts of serum and exogenous cytokines. We aimed to develop an expansion protocol for NK-92 cells in an effort to generate a cost-efficient, xeno-free, clinical grade manufactured master cell line for therapeutic applications. By making functional assays with NK-92 cells cultured under serum-free conditions (NK-92SF) and comparing to serum-supplemented NK-92 cells (NK-92S) we did not observe significant alterations in the viability, proliferation, receptor expression levels, or in perforin and granzyme levels. Interestingly, even though NK-92SF cells displayed decreased degranulation and cytotoxicity against tumor cells in vitro, the degranulation capacity was recovered after overnight incubation with 20% serum in the medium. Moreover, lentiviral vector-based genetic modification efficiency of NK-92SF cells was comparable with NK-92S cells. The application of similar strategies can be useful in reducing the costs of manufacturing cells for clinical use and can help us understand and implement strategies towards chemically defined expansion and genetic modification protocols.
Keywords: NK cell; NK-92; immunotherapy; serum-free.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Robertson M.J., Cochran K.J., Cameron C., Le J.M., Tantravahi R., Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 1996;24:406–415. - PubMed
-
- Gong J.H., Maki G., Klingemann H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

