Genome-Wide Characterization and Identification of Trihelix Transcription Factor and Expression Profiling in Response to Abiotic Stresses in Rice (Oryza sativa L.)
- PMID: 30634597
- PMCID: PMC6358761
- DOI: 10.3390/ijms20020251
Genome-Wide Characterization and Identification of Trihelix Transcription Factor and Expression Profiling in Response to Abiotic Stresses in Rice (Oryza sativa L.)
Abstract
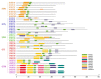
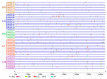
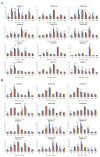
Trihelix transcription factors play a role in plant growth, development and various stress responses. Here, we identified 41 trihelix family genes in the rice genome. These OsMSLs (Myb/SANT-LIKE) were located on twelve chromosomes. Synteny analysis indicated only six duplicated gene pairs in the rice trihelix family. Phylogenetic analysis of these OsMSLs and the trihelix genes from other species divided them into five clusters. OsMSLs from different groups significantly diverged in terms of gene structure and conserved functional domains. However, all OsMSLs contained the same five cis-elements. Some of these were responsive to light and dehydration stress. All OsMSLs expressed in four tissues and six developmental stages of rice but with different expression patterns. Quantitative real-time PCR analysis revealed that the OsMSLs responded to abiotic stresses including drought and high salt stress and stress signal molecule including ABA (abscisic acid), hydrogen peroxide. OsMSL39 were simultaneously expressed under all treatments, while OsMSL28 showed high expression under hydrogen peroxide, drought, and high salt treatments. Moreover, OsMSL16/27/33 displayed significant expression under ABA and drought treatments. Nevertheless, their responses were regulated by light. The expression levels of the 12 chosen OsMSLs differed between light and dark conditions. In conclusion, our results helped elucidate the biological functions of rice trihelix genes and provided a theoretical basis for further characterizing their biological roles in responding to abiotic stresses.
Keywords: light; phylogenetic analysis; rice; stress response; trihelix transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

