Glutamine Addiction and Therapeutic Strategies in Lung Cancer
- PMID: 30634602
- PMCID: PMC6359540
- DOI: 10.3390/ijms20020252
Glutamine Addiction and Therapeutic Strategies in Lung Cancer
Abstract
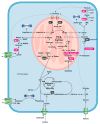
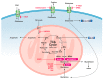
Lung cancer cells are well-documented to rewire their metabolism and energy production networks to support rapid survival and proliferation. This metabolic reorganization has been recognized as a hallmark of cancer. The increased uptake of glucose and the increased activity of the glycolytic pathway have been extensively described. However, over the past years, increasing evidence has shown that lung cancer cells also require glutamine to fulfill their metabolic needs. As a nitrogen source, glutamine contributes directly (or indirectly upon conversion to glutamate) to many anabolic processes in cancer, such as the biosynthesis of amino acids, nucleobases, and hexosamines. It plays also an important role in the redox homeostasis, and last but not least, upon conversion to α-ketoglutarate, glutamine is an energy and anaplerotic carbon source that replenishes tricarboxylic acid cycle intermediates. The latter is generally indicated as glutaminolysis. In this review, we explore the role of glutamine metabolism in lung cancer. Because lung cancer is the leading cause of cancer death with limited curative treatment options, we focus on the potential therapeutic approaches targeting the glutamine metabolism in cancer.
Keywords: Lung cancer; glutamine; glutaminolysis; metabolism; pathways; targeted treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Chen W., Zu Y., Huang Q., Chen F., Wang G., Lan W., Bai C., Lu S., Yue Y., Deng F. Study on metabonomic characteristics of human lung cancer using high resolution magic-angle spinning 1h nmr spectroscopy and multivariate data analysis. Magn. Reson. Med. 2011;66:1531–1540. doi: 10.1002/mrm.22957. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

