The Roles of Ubiquitin-Binding Protein Shuttles in the Degradative Fate of Ubiquitinated Proteins in the Ubiquitin-Proteasome System and Autophagy
- PMID: 30634694
- PMCID: PMC6357184
- DOI: 10.3390/cells8010040
The Roles of Ubiquitin-Binding Protein Shuttles in the Degradative Fate of Ubiquitinated Proteins in the Ubiquitin-Proteasome System and Autophagy
Abstract
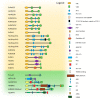
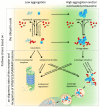
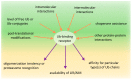
The ubiquitin-proteasome system (UPS) and autophagy are the two major intracellular protein quality control (PQC) pathways that are responsible for cellular proteostasis (homeostasis of the proteome) by ensuring the timely degradation of misfolded, damaged, and unwanted proteins. Ubiquitination serves as the degradation signal in both these systems, but substrates are precisely targeted to one or the other pathway. Determining how and when cells target specific proteins to these two alternative PQC pathways and control the crosstalk between them are topics of considerable interest. The ubiquitin (Ub) recognition code based on the type of Ub-linked chains on substrate proteins was believed to play a pivotal role in this process, but an increasing body of evidence indicates that the PQC pathway choice is also made based on other criteria. These include the oligomeric state of the Ub-binding protein shuttles, their conformation, protein modifications, and the presence of motifs that interact with ATG8/LC3/GABARAP (autophagy-related protein 8/microtubule-associated protein 1A/1B-light chain 3/GABA type A receptor-associated protein) protein family members. In this review, we summarize the current knowledge regarding the Ub recognition code that is bound by Ub-binding proteasomal and autophagic receptors. We also discuss how cells can modify substrate fate by modulating the structure, conformation, and physical properties of these receptors to affect their shuttling between both degradation pathways.
Keywords: protein quality control; proteostasis; selective autophagy; ubiquitin-proteasome system; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures