Structures, Activities and Drug-Likeness of Anti-Infective Xanthone Derivatives Isolated from the Marine Environment: A Review
- PMID: 30634698
- PMCID: PMC6359551
- DOI: 10.3390/molecules24020243
Structures, Activities and Drug-Likeness of Anti-Infective Xanthone Derivatives Isolated from the Marine Environment: A Review
Abstract
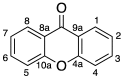
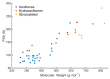
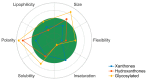
Marine organisms represent almost half of total biodiversity and are a very important source of new bioactive substances. Within the varied biological activities found in marine products, their antimicrobial activity is one of the most relevant. Infectious diseases are responsible for high levels of morbidity and mortality and many antimicrobials lose their effectiveness with time due to the development of resistance. These facts justify the high importance of finding new, effective and safe anti-infective agents. Among the variety of biological activities of marine xanthone derivatives, one that must be highlighted is their anti-infective properties. In this work, a literature review of marine xanthones with anti-infective activity, namely antibacterial, antifungal, antiparasitic and antiviral, is presented. Their structures, biological activity, sources and the methods used for bioactivity evaluation are described. The xanthone derivatives are grouped in three sets: xanthones, hydroxanthones and glycosylated derivatives. Moreover, molecular descriptors, biophysico-chemical properties, and pharmacokinetic parameters were calculated, and the chemical space occupied by marine xanthone derivatives is recognized. The chemical space was compared with marketed drugs and framed accordingly to the drug-likeness concept in order to profile the pharmacokinetic of anti-infective marine xanthone derivatives.
Keywords: ADME; antimicrobial; marine; physicochemical properties; xanthones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

