From Aggregates to Porous Three-Dimensional Scaffolds through a Mechanochemical Approach to Design Photosensitive Chitosan Derivatives
- PMID: 30634710
- PMCID: PMC6356335
- DOI: 10.3390/md17010048
From Aggregates to Porous Three-Dimensional Scaffolds through a Mechanochemical Approach to Design Photosensitive Chitosan Derivatives
Abstract
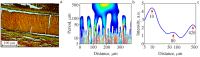
The crustacean processing industry produces large quantities of waste by-products (up to 70%). Such wastes could be used as raw materials for producing chitosan, a polysaccharide with a unique set of biochemical properties. However, the preparation methods and the long-term stability of chitosan-based products limit their application in biomedicine. In this study, different scale structures, such as aggregates, photo-crosslinked films, and 3D scaffolds based on mechanochemically-modified chitosan derivatives, were successfully formed. Dynamic light scattering revealed that aggregation of chitosan derivatives becomes more pronounced with an increase in the number of hydrophobic substituents. Although the results of the mechanical testing revealed that the plasticity of photo-crosslinked films was 5⁻8% higher than that for the initial chitosan films, their tensile strength remained unchanged. Different types of polymer scaffolds, such as flexible and porous ones, were developed by laser stereolithography. In vivo studies of the formed structures showed no dystrophic and necrobiotic changes, which proves their biocompatibility. Moreover, the wavelet analysis was used to show that the areas of chitosan film degradation were periodic. Comparing the results of the wavelet analysis and X-ray diffraction data, we have concluded that degradation occurs within less ordered amorphous regions in the polymer bulk.
Keywords: chitosan; laser stereolithography; long-term stability; mechanochemical synthesis; scaffold; tissue reaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Synthesis, Characterization, and Histological Evaluation of Chitosan-Ruta Graveolens Essential Oil Films.Molecules. 2020 Apr 7;25(7):1688. doi: 10.3390/molecules25071688. Molecules. 2020. PMID: 32272702 Free PMC article.
-
Mechanical response of porous scaffolds for cartilage engineering.Physiol Res. 2007;56 Suppl 1:S17-S25. doi: 10.33549/physiolres.931297. Epub 2007 May 31. Physiol Res. 2007. PMID: 17552899
-
Genipin-crosslinked silk fibroin/hydroxybutyl chitosan nanofibrous scaffolds for tissue-engineering application.J Biomed Mater Res A. 2010 Dec 1;95(3):870-81. doi: 10.1002/jbm.a.32895. J Biomed Mater Res A. 2010. PMID: 20824649
-
Design and fabrication of porous chitosan scaffolds with tunable structures and mechanical properties.Carbohydr Polym. 2017 Dec 1;177:210-216. doi: 10.1016/j.carbpol.2017.08.069. Epub 2017 Aug 30. Carbohydr Polym. 2017. PMID: 28962760
-
Processing and characterization of chitosan/PVA and methylcellulose porous scaffolds for tissue engineering.Mater Sci Eng C Mater Biol Appl. 2016 Apr 1;61:484-91. doi: 10.1016/j.msec.2015.12.084. Epub 2015 Dec 30. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26838875
Cited by
-
Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis.Polymers (Basel). 2020 Jun 22;12(6):1397. doi: 10.3390/polym12061397. Polymers (Basel). 2020. PMID: 32580366 Free PMC article. Review.
-
A defined road to tracheal reconstruction: laser structuring and cell support for rapid clinic translation.Stem Cell Res Ther. 2022 Jul 16;13(1):317. doi: 10.1186/s13287-022-02997-8. Stem Cell Res Ther. 2022. PMID: 35842689 Free PMC article.
-
Mechanochemical Transformations of Polysaccharides: A Systematic Review.Int J Mol Sci. 2022 Sep 9;23(18):10458. doi: 10.3390/ijms231810458. Int J Mol Sci. 2022. PMID: 36142370 Free PMC article.
-
Extraction of Hydroxyapatite Nanostructures from Marine Wastes for the Fabrication of Biopolymer-Based Porous Scaffolds.Mar Drugs. 2019 Dec 27;18(1):26. doi: 10.3390/md18010026. Mar Drugs. 2019. PMID: 31892123 Free PMC article.
-
Application of Bioactive Hydrogels for Functional Treatment of Intrauterine Adhesion.Front Bioeng Biotechnol. 2021 Sep 21;9:760943. doi: 10.3389/fbioe.2021.760943. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34621732 Free PMC article. Review.
References
-
- Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013;49:780–792. doi: 10.1016/j.eurpolymj.2012.12.009. - DOI
-
- Thapa B., Narain R. Mechanism, current challenges and new approaches for non viral gene delivery. Polym. Nanomater. Gene Ther. 2016:1–27. doi: 10.1016/B978-0-08-100520-0.00001-1. - DOI
MeSH terms
Substances
Grants and funding
- 15-15-00132 (formation of film and three-dimensional samples, their characterization)/Russian Science Foundation
- 18-32-00222 (laser stereolithography of hydrogel matrices)/Russian Foundation for Basic Research
- (in vivo and morphological studies, the development of a photosensitive composition)/Russian academic excellence project '5-100'
- (wavelet analysis of samples)/Ministry of Science and Higher Education within the State assignment FSRC «Crystallography and Photonics» RAS
LinkOut - more resources
Full Text Sources

