Generational conservation of composition and diversity of field-acquired midgut microbiota in Anopheles gambiae (sensu lato) during colonization in the laboratory
- PMID: 30635018
- PMCID: PMC6329181
- DOI: 10.1186/s13071-019-3287-0
Generational conservation of composition and diversity of field-acquired midgut microbiota in Anopheles gambiae (sensu lato) during colonization in the laboratory
Abstract
Background: The gut microbiota is known to play a role in a mosquito vector's life history, a subject of increasing research. Laboratory experiments are essential for such studies and require laboratory colonies. In this study, the conservation of field-obtained midgut microbiota was evaluated in laboratory-reared Anopheles gambiae (s.l.) mosquitoes continuously hatched in water from field breeding habitats.
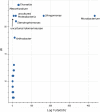
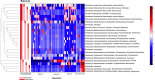
Methods: Pupae and late instars were obtained from the field and reared, and the emerged adults were blood-fed. The eggs obtained from them were hatched in either water from the field or in dechlorinated tap water. The mosquito colonies were maintained for 10 generations. Midguts of female adults from unfed F0 (emerging from field-caught pupae and larvae), F5 and F10 were dissected out and genomic DNA was extracted for 16S metagenomic sequencing. The sequences were compared to investigate the diversity and bacterial compositional differences using ANCOM and correlation clustering methods.
Results: Less than 10% of the bacterial families identified had differential relative abundances between generational groups and accounted for 46% of the variation observed. Although diversity reduced in F10 mosquitoes during laboratory colonization (Shannon-Weaver; P-value < 0.05), 50% of bacterial genera were conserved in those bred continuously in field-water compared to 38% in those bred in dechlorinated tap water.
Conclusions: To our knowledge, this study is the first report on the assessment of gut bacterial community of mosquitoes during laboratory colonization and recommends the use of water from the natural breeding habitats if they are intended for microbiota research.
Keywords: Anopheles gambiae (sensu lato); Breeding habitat; Field water; Laboratory colonization; Midgut microbiota.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- WHO . Global vector control response 2017–2030. Geneva: World Health Organization; 2017. p. 52.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

