Bovine endometrial MSC: mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation
- PMID: 30635057
- PMCID: PMC6330450
- DOI: 10.1186/s13287-018-1129-1
Bovine endometrial MSC: mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation
Abstract
Background: The uterus is a histologically dynamic organ, and the mechanisms coordinating its regeneration during the oestrous cycle and implantation are poorly understood. The aim of this study was to isolate, immortalize and characterize bovine endometrial mesenchymal stem cell (eMSC) lines from different oestrous cycle stages (embryo in the oviduct, embryo in the uterus or absence of embryo) and examine their migratory and immunomodulatory properties in an inflammatory or implantation-like environment, as well as possible changes in cell transdifferentiation.
Methods: eMSCs were isolated and analysed in terms of morphological features, expression of cell surface and intracellular markers of pluripotency, inmunocytochemical analyses, alkaline phosphatase activity, proliferation and osteogenic or chondrogenic differentiation capacities, as well as their ability to migrate in response to inflammatory (TNF-α or IL-1β) or implantation (IFN-τ) cytokines and their immunomodulatory effect in the proliferation of T cells.
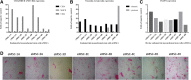
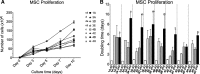
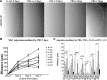
Results: All eMSCs showed MSC properties such as adherence to plastic, high proliferative capacity, expression of CD44 and vimentin, undetectable expression of CD34 or MHCII, positivity for Pou5F1 and alkaline phosphatase activity. In the absence of an embryo, eMSC showed an apparent mesenchymal to epithelial transition state. eMSC during the entire oestrous cycle differentiated to osteogenic or chondrogenic lineages, showed the ability to suppress T cell proliferation and showed migratory capacity towards pro-inflammatory signal, while responded with a block in their migration to the embryo-derived pregnancy signal.
Conclusion: This study describes for the first time the isolation, immortalization and characterization of bovine mesenchymal stem cell lines from different oestrous cycle stages, with a clear mesenchymal pattern and immunomodulatory properties. Our study also reports that the migratory capacity of the eMSC was increased towards an inflammatory niche but was reduced in response to the expression of implantation cytokine by the embryo. The combination of both signals (pro-inflammatory and implantation) would ensure the retention of eMSC in case of pregnancy, to ensure the immunomodulation necessary in the mother for embryo survival. In addition, in the absence of an embryo, eMSC showed an apparent mesenchymal to epithelial transition state.
Keywords: Cell migration; Embryo implantation; Endometrial mesenchymal stem cells; Inflammation.
Conflict of interest statement
Ethics approval and consent to participate
Whole blood, used as source of T lymphocytes for in vitro allogenenic proliferation experiments, was obtained from healthy donors after informed consent approved by the Germans Trias i Pujol Universitary Hospital Ethics Committee. This article does not include any individual patient’s data.
Heifer uterus and ovaries were collected from routine slaughtered at the local abattoir.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Mesenchymal Stem Cells in Embryo-Maternal Communication under Healthy Conditions or Viral Infections: Lessons from a Bovine Model.Cells. 2022 Jun 7;11(12):1858. doi: 10.3390/cells11121858. Cells. 2022. PMID: 35740987 Free PMC article. Review.
-
Iberian pig mesenchymal stem/stromal cells from dermal skin, abdominal and subcutaneous adipose tissues, and peripheral blood: in vitro characterization and migratory properties in inflammation.Stem Cell Res Ther. 2018 Jul 4;9(1):178. doi: 10.1186/s13287-018-0933-y. Stem Cell Res Ther. 2018. PMID: 29973295 Free PMC article.
-
Bovine peripheral blood MSCs chemotax towards inflammation and embryo implantation stimuli.J Cell Physiol. 2021 Feb;236(2):1054-1067. doi: 10.1002/jcp.29915. Epub 2020 Jul 2. J Cell Physiol. 2021. PMID: 32617972
-
Human Endometrial Fibroblasts Derived from Mesenchymal Progenitors Inherit Progesterone Resistance and Acquire an Inflammatory Phenotype in the Endometrial Niche in Endometriosis.Biol Reprod. 2016 May;94(5):118. doi: 10.1095/biolreprod.115.136010. Epub 2016 Apr 13. Biol Reprod. 2016. PMID: 27075616 Free PMC article.
-
The role of mesenchymal-epithelial transition in endometrial function.Hum Reprod Update. 2019 Jan 1;25(1):114-133. doi: 10.1093/humupd/dmy035. Hum Reprod Update. 2019. PMID: 30407544 Review.
Cited by
-
Conceptus-modulated innate immune function during early pregnancy in ruminants: a review.Anim Reprod. 2021 May 10;18(1):e20200048. doi: 10.1590/1984-3143-AR2020-0048. Anim Reprod. 2021. PMID: 34122650 Free PMC article. Review.
-
Embryonic Trophectoderm Secretomics Reveals Chemotactic Migration and Intercellular Communication of Endometrial and Circulating MSCs in Embryonic Implantation.Int J Mol Sci. 2021 May 26;22(11):5638. doi: 10.3390/ijms22115638. Int J Mol Sci. 2021. PMID: 34073234 Free PMC article.
-
Isolation and characterization mesenchymal stem cells from red panda (Ailurus fulgens styani) endometrium.Conserv Physiol. 2022 Feb 21;10(1):coac004. doi: 10.1093/conphys/coac004. eCollection 2022 Jan 1. Conserv Physiol. 2022. PMID: 35211318 Free PMC article.
-
Multilineage Differentiation Potential of Equine Adipose-Derived Stromal/Stem Cells from Different Sources.Animals (Basel). 2023 Apr 15;13(8):1352. doi: 10.3390/ani13081352. Animals (Basel). 2023. PMID: 37106915 Free PMC article.
-
Mesenchymal Stem Cells in Embryo-Maternal Communication under Healthy Conditions or Viral Infections: Lessons from a Bovine Model.Cells. 2022 Jun 7;11(12):1858. doi: 10.3390/cells11121858. Cells. 2022. PMID: 35740987 Free PMC article. Review.
References
-
- Hue I, Degrelle SA, Campion E, Renard JP. Gene expression in elongating and gastrulating embryos from ruminants. Soc Reprod Fertil Suppl. 2007;64:365–377. - PubMed
-
- Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci. 2004;82(E-Suppl):E4–13. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

