Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial
- PMID: 30635141
- PMCID: PMC6356450
- DOI: 10.1016/S0140-6736(18)32221-9
Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial
Abstract
Background: Infections acquired in hospital are an important cause of morbidity and mortality in very preterm infants. Several small trials have suggested that supplementing the enteral diet of very preterm infants with lactoferrin, an antimicrobial protein processed from cow's milk, prevents infections and associated complications. The aim of this large randomised controlled trial was to collect data to enhance the validity and applicability of the evidence from previous trials to inform practice.
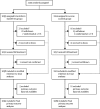
Methods: In this randomised placebo-controlled trial, we recruited very preterm infants born before 32 weeks' gestation in 37 UK hospitals and younger than 72 h at randomisation. Exclusion criteria were presence of a severe congenital anomaly, anticipated enteral fasting for longer than 14 days, or no realistic prospect of survival. Eligible infants were randomly assigned (1:1) to receive either enteral bovine lactoferrin (150 mg/kg per day; maximum 300 mg/day; lactoferrin group) or sucrose (same dose; control group) once daily until 34 weeks' postmenstrual age. Web-based randomisation minimised for recruitment site, gestation (completed weeks), sex, and single versus multifetal pregnancy. Parents, caregivers, and outcome assessors were unaware of group assignment. The primary outcome was microbiologically confirmed or clinically suspected late-onset infection (occurring >72 h after birth), which was assessed in all participants for whom primary outcome data was available by calculating the relative risk ratio with 95% CI between the two groups. The trial is registered with the International Standard Randomised Controlled Trial Number 88261002.
Findings: We recruited 2203 participants between May 7, 2014, and Sept 28, 2017, of whom 1099 were assigned to the lactoferrin group and 1104 to the control group. Four infants had consent withdrawn or unconfirmed, leaving 1098 infants in the lactoferrin group and 1101 in the sucrose group. Primary outcome data for 2182 infants (1093 [99·5%] of 1098 in the lactoferrin group and 1089 [99·0] of 1101 in the control group) were available for inclusion in the modified intention-to-treat analyses. 316 (29%) of 1093 infants in the intervention group acquired a late-onset infection versus 334 (31%) of 1089 in the control group. The risk ratio adjusted for minimisation factors was 0·95 (95% CI 0·86-1·04; p=0·233). During the trial there were 16 serious adverse events for infants in the lactoferrin group and 10 for infants in the control group. Two events in the lactoferrin group (one case of blood in stool and one death after intestinal perforation) were assessed as being possibly related to the trial intervention.
Interpretation: Enteral supplementation with bovine lactoferrin does not reduce the risk of late-onset infection in very preterm infants. These data do not support its routine use to prevent late-onset infection and associated morbidity or mortality in very preterm infants.
Funding: UK National Institute for Health Research Health Technology Assessment programme (10/57/49).
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Does bovine lactoferrin prevent late-onset neonatal sepsis?Lancet. 2019 Feb 2;393(10170):382-384. doi: 10.1016/S0140-6736(18)32390-0. Epub 2019 Jan 8. Lancet. 2019. PMID: 30635140 No abstract available.
References
-
- Duley L, Uhm S, Oliver S, Preterm Birth Priority Setting Partnership Steering Group Top 15 UK research priorities for preterm birth. Lancet. 2014;383:2041–2042. - PubMed
-
- Cailes B, Kortsalioudaki C, Buttery J. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed. 2017;103:F547–F553. - PubMed
-
- Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390:1770–1780. - PubMed
-
- Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics. 2004;114:348–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

