Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies
- PMID: 30635225
- PMCID: PMC6379893
- DOI: 10.1016/S2213-8587(18)30313-9
Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies
Abstract
Background: Change in albuminuria as a surrogate endpoint for progression of chronic kidney disease is strongly supported by biological plausibility, but empirical evidence to support its validity in epidemiological studies is lacking. We aimed to assess the consistency of the association between change in albuminuria and risk of end-stage kidney disease in a large individual participant-level meta-analysis of observational studies.
Methods: In this meta-analysis, we collected individual-level data from eligible cohorts in the Chronic Kidney Disease Prognosis Consortium (CKD-PC) with data on serum creatinine and change in albuminuria and more than 50 events on outcomes of interest. Cohort data were eligible if participants were aged 18 years or older, they had a repeated measure of albuminuria during an elapsed period of 8 months to 4 years, subsequent end-stage kidney disease or mortality follow-up data, and the cohort was active during this consortium phase. We extracted participant-level data and quantified percentage change in albuminuria, measured as change in urine albumin-to-creatinine ratio (ACR) or urine protein-to-creatinine ratio (PCR), during baseline periods of 1, 2, and 3 years. Our primary outcome of interest was development of end-stage kidney disease after a baseline period of 2 years. We defined an end-stage kidney disease event as initiation of kidney replacement therapy. We quantified associations of percentage change in albuminuria with subsequent end-stage kidney disease using Cox regression in each cohort, followed by random-effects meta-analysis. We further adjusted for regression dilution to account for imprecision in the estimation of albuminuria at the participant level. We did multiple subgroup analyses, and also repeated our analyses using participant-level data from 14 clinical trials, including nine clinical trials not in CKD-PC.
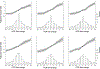
Findings: Between July, 2015, and June, 2018, we transferred and analysed data from 28 cohorts in the CKD-PC, which included 693 816 individuals (557 583 [80%] with diabetes). Data for 675 904 individuals and 7461 end-stage kidney disease events were available for our primary outcome analysis. Change in ACR was consistently associated with subsequent risk of end-stage kidney disease. The adjusted hazard ratio (HR) for end-stage kidney disease after a 30% decrease in ACR during a baseline period of 2 years was 0·83 (95% CI 0·74-0·94), decreasing to 0·78 (0·66-0·92) after further adjustment for regression dilution. Adjusted HRs were fairly consistent across cohorts and subgroups (ie, estimated glomerular filtration rate, diabetes, and sex), but the association was somewhat stronger among participants with higher baseline ACR than among those with lower baseline ACR (pinteraction<0·0001). In individuals with baseline ACR of 300 mg/g or higher, a 30% decrease in ACR over 2 years was estimated to confer a more than 1% absolute reduction in 10-year risk of end-stage kidney disease, even at early stages of chronic kidney disease. Results were generally similar when we used change in PCR and when study populations from clinical trials were assessed.
Interpretation: Change in albuminuria was consistently associated with subsequent risk of end-stage kidney disease across a range of cohorts, lending support to the use of change in albuminuria as a surrogate endpoint for end-stage kidney disease in clinical trials of progression of chronic kidney disease in the setting of increased albuminuria.
Funding: US National Kidney Foundation and US National Institute of Diabetes and Digestive and Kidney Diseases.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


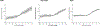
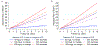
Comment in
-
Working towards novel albuminuria endpoints in chronic kidney disease.Lancet Diabetes Endocrinol. 2019 Feb;7(2):80-82. doi: 10.1016/S2213-8587(18)30352-8. Epub 2019 Jan 8. Lancet Diabetes Endocrinol. 2019. PMID: 30635227 No abstract available.
-
Albuminuria: a target for clinical trials in kidney disease?Nat Rev Nephrol. 2019 May;15(5):257-258. doi: 10.1038/s41581-019-0123-x. Nat Rev Nephrol. 2019. PMID: 30765852 No abstract available.
-
Change in albuminuria as a surrogate endpoint in chronic kidney disease.Lancet Diabetes Endocrinol. 2019 May;7(5):335. doi: 10.1016/S2213-8587(19)30089-0. Lancet Diabetes Endocrinol. 2019. PMID: 31003620 No abstract available.
-
Change in albuminuria as a surrogate endpoint in chronic kidney disease.Lancet Diabetes Endocrinol. 2019 May;7(5):335-336. doi: 10.1016/S2213-8587(19)30085-3. Lancet Diabetes Endocrinol. 2019. PMID: 31003621 No abstract available.
-
Change in albuminuria as a surrogate endpoint in chronic kidney disease - Authors' reply.Lancet Diabetes Endocrinol. 2019 May;7(5):336-337. doi: 10.1016/S2213-8587(19)30080-4. Lancet Diabetes Endocrinol. 2019. PMID: 31003622 No abstract available.
References
-
- Parving HH, Andersen AR, Smidt UM, Friisberg B, Svendsen PA. Reduced albuminuria during early and aggressive antihypertensive treatment of insulin-dependent diabetic patients with diabetic nephropathy. Diabetes Care Jul-Aug 1981;4(4):459–463. - PubMed
-
- de Zeeuw D Should albuminuria be a therapeutic target in patients with hypertension and diabetes? Am J Hypertens November 2004;17(11 Pt 2):11S–15S; quiz A12–14. - PubMed
-
- Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis December 2014;64(6):821–835. - PubMed
-
- Heerspink H, Tom G, Tighiouart H, et al. Change in Albuminuria as a Surrogate End Point for Kidney Disease Progression in Clinical Trials: A Meta-analysis of Treatment Effects of 41 Randomized Trials. Lancet Diabetes Endocrinol 2018;(in press). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

