Oligoadenylate-Synthetase-Family Protein OASL Inhibits Activity of the DNA Sensor cGAS during DNA Virus Infection to Limit Interferon Production
- PMID: 30635239
- PMCID: PMC6342484
- DOI: 10.1016/j.immuni.2018.12.013
Oligoadenylate-Synthetase-Family Protein OASL Inhibits Activity of the DNA Sensor cGAS during DNA Virus Infection to Limit Interferon Production
Abstract
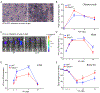
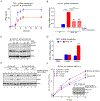
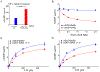
Interferon-inducible human oligoadenylate synthetase-like (OASL) and its mouse ortholog, Oasl2, enhance RNA-sensor RIG-I-mediated type I interferon (IFN) induction and inhibit RNA virus replication. Here, we show that OASL and Oasl2 have the opposite effect in the context of DNA virus infection. In Oasl2-/- mice and OASL-deficient human cells, DNA viruses such as vaccinia, herpes simplex, and adenovirus induced increased IFN production, which resulted in reduced virus replication and pathology. Correspondingly, ectopic expression of OASL in human cells inhibited IFN induction through the cGAS-STING DNA-sensing pathway. cGAS was necessary for the reduced DNA virus replication observed in OASL-deficient cells. OASL directly and specifically bound to cGAS independently of double-stranded DNA, resulting in a non-competitive inhibition of the second messenger cyclic GMP-AMP production. Our findings define distinct mechanisms by which OASL differentially regulates host IFN responses during RNA and DNA virus infection and identify OASL as a negative-feedback regulator of cGAS.
Keywords: DNA virus; IFN; OASL; cGAMP; cGAS; transcriptional signalling.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTEREST
PS and SHT are full time employees and may have ownership interests at Western Oncolytics at present. However, the work presented here were carried out while they were at the University of Pittsburgh and no part of this work was carried out at the company.
Figures



Similar articles
-
Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor.Immunity. 2014 Jun 19;40(6):936-48. doi: 10.1016/j.immuni.2014.05.007. Epub 2014 Jun 12. Immunity. 2014. PMID: 24931123 Free PMC article.
-
The Association of OASL and Type I Interferons in the Pathogenesis and Survival of Intracellular Replicating Bacterial Species.Front Cell Infect Microbiol. 2017 May 19;7:196. doi: 10.3389/fcimb.2017.00196. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28580319 Free PMC article. Review.
-
The two faces of oligoadenylate synthetase-like: effective antiviral protein and negative regulator of innate immunity.Curr Opin Virol. 2023 Jun;60:101329. doi: 10.1016/j.coviro.2023.101329. Epub 2023 Apr 19. Curr Opin Virol. 2023. PMID: 37079941 Review.
-
OASs in Defense of Mycobacterial Infection: Angels or Demons?Curr Issues Mol Biol. 2021;40:221-230. doi: 10.21775/cimb.040.221. Epub 2020 Jul 1. Curr Issues Mol Biol. 2021. PMID: 32609093 Review.
-
Interferon-Inducible Oligoadenylate Synthetase-Like Protein Acts as an Antiviral Effector against Classical Swine Fever Virus via the MDA5-Mediated Type I Interferon-Signaling Pathway.J Virol. 2017 May 12;91(11):e01514-16. doi: 10.1128/JVI.01514-16. Print 2017 Jun 1. J Virol. 2017. PMID: 28331099 Free PMC article.
Cited by
-
The role of 2'-5'-oligoadenylate synthase-like protein (OASL1) in biliary and hepatotoxin-induced liver injury in mice.Sci Rep. 2024 Sep 19;14(1):21873. doi: 10.1038/s41598-024-72465-1. Sci Rep. 2024. PMID: 39300174 Free PMC article.
-
Elucidating the transcriptomic response of adult-derived mHypoA-2/12 mouse hypothalamic neuron cell line to cannabidiol (CBD) exposure.J Appl Genet. 2025 May 8. doi: 10.1007/s13353-025-00970-8. Online ahead of print. J Appl Genet. 2025. PMID: 40335839
-
Oligoadenylate synthetase-like aggravated Newcastle disease virus-induced necroptosis in glioma cells.Front Oncol. 2025 Apr 11;15:1574214. doi: 10.3389/fonc.2025.1574214. eCollection 2025. Front Oncol. 2025. PMID: 40330823 Free PMC article.
-
Clinical and Functional Characterization of CD-NTase Enzymes in Esophageal Squamous Cell Carcinoma.J Cancer. 2025 Jun 12;16(9):2822-2836. doi: 10.7150/jca.100226. eCollection 2025. J Cancer. 2025. PMID: 40657359 Free PMC article.
-
2'-5' oligoadenylate synthetase‑like 1 (OASL1) protects against atherosclerosis by maintaining endothelial nitric oxide synthase mRNA stability.Nat Commun. 2022 Nov 4;13(1):6647. doi: 10.1038/s41467-022-34433-z. Nat Commun. 2022. PMID: 36333342 Free PMC article.
References
-
- Andreeva L, Hiller B, Kostrewa D, Lässig C, de Oliveira Mann CC, Jan Drexler D, Maiser A, Gaidt M, Leonhardt H, Hornung V, et al. (2017). cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein–DNA ladders. Nature 549, 394–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

