Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury
- PMID: 30635296
- PMCID: PMC6437604
- DOI: 10.1183/13993003.00786-2018
Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury
Abstract
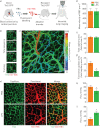
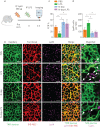
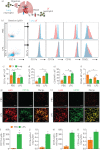
The lung is highly vulnerable during sepsis, yet its functional deterioration accompanied by disturbances in the pulmonary microcirculation is poorly understood. This study aimed to investigate how the pulmonary microcirculation is distorted in sepsis-induced acute lung injury (ALI) and reveal the underlying cellular pathophysiologic mechanism.Using a custom-made intravital lung microscopic imaging system in a murine model of sepsis-induced ALI, we achieved direct real-time visualisation of the pulmonary microcirculation and circulating cells in vivo We derived the functional capillary ratio (FCR) as a quantitative parameter for assessing the fraction of functional microvasculature in the pulmonary microcirculation and dead space.We identified that the FCR rapidly decreases in the early stage of sepsis-induced ALI. The intravital imaging revealed that this decrease resulted from the generation of dead space, which was induced by prolonged neutrophil entrapment within the capillaries. We further showed that the neutrophils had an extended sequestration time and an arrest-like dynamic behaviour, both of which triggered neutrophil aggregates inside the capillaries and arterioles. Finally, we found that Mac-1 (CD11b/CD18) was upregulated in the sequestered neutrophils and that a Mac-1 inhibitor restored the FCR and improved hypoxaemia.Using the intravital lung imaging system, we observed that Mac-1-upregulated neutrophil aggregates led to the generation of dead space in the pulmonary microcirculation that was recovered by a Mac-1 inhibitor in sepsis-induced ALI.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: I. Park has nothing to disclose. Conflict of interest: M. Kim has nothing to disclose. Conflict of interest: K. Choe has nothing to disclose. Conflict of interest: E. Song has nothing to disclose. Conflict of interest: H. Seo has nothing to disclose. Conflict of interest: Y. Hwang has nothing to disclose. Conflict of interest: J. Ahn has nothing to disclose. Conflict of interest: S-H. Lee has nothing to disclose. Conflict of interest: J.H. Lee has nothing to disclose. Conflict of interest: Y.H. Jo has nothing to disclose. Conflict of interest: K. Kim has nothing to disclose. Conflict of interest: G.Y. Koh has nothing to disclose. Conflict of interest: P. Kim has nothing to disclose.
Figures




References
-
- Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013: statistical brief #204. In:Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD, Agency for Healthcare Research and Quality (US), 2016. - PubMed
-
- Hall MJ, Levant S, DeFrances CJ. Trends in inpatient hospital deaths: National Hospital Discharge Survey, 2000–2010. NCHS Data Brief 2013; 118:1–8. - PubMed
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369: 840–851. - PubMed
-
- Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012; 40: 754–761. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
