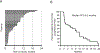Autophagy Inhibition to Augment mTOR Inhibition: a Phase I/II Trial of Everolimus and Hydroxychloroquine in Patients with Previously Treated Renal Cell Carcinoma
- PMID: 30635337
- PMCID: PMC8915191
- DOI: 10.1158/1078-0432.CCR-18-2204
Autophagy Inhibition to Augment mTOR Inhibition: a Phase I/II Trial of Everolimus and Hydroxychloroquine in Patients with Previously Treated Renal Cell Carcinoma
Abstract
Purpose: Everolimus inhibits the mTOR, activating cytoprotective autophagy. Hydroxychloroquine inhibits autophagy. On the basis of preclinical data demonstrating synergistic cytotoxicity when mTOR inhibitors are combined with an autophagy inhibitor, we launched a clinical trial of combined everolimus and hydroxychloroquine, to determine its safety and activity in patients with clear-cell renal cell carcinoma (ccRCC).
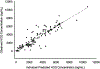
Patients and methods: Three centers conducted a phase I/II trial of everolimus 10 mg daily and hydroxychloroquine in patients with advanced ccRCC. The objectives were to determine the MTD of hydroxychloroquine with daily everolimus, and to estimate the rate of 6-month progression-free survival (PFS) in patients with ccRCC receiving everolimus/hydroxychloroquine after 1-3 prior treatment regimens. Correlative studies to identify patient subpopulations that achieved the most benefit included population pharmacokinetics, measurement of autophagosomes by electron microscopy, and next-generation tumor sequencing.
Results: No dose-limiting toxicity was observed in the phase I trial. The recommended phase II dose of hydroxychloroquine 600 mg twice daily with everolimus was identified. Disease control [stable disease + partial response (PR)] occurred in 22 of 33 (67%) evaluable patients. PR was observed in 2 of 33 patients (6%). PFS ≥ 6 months was achieved in 15 of 33 (45%) of patients who achieved disease control.
Conclusions: Combined hydroxychloroquine 600 mg twice daily with 10 mg daily everolimus was tolerable. The primary endpoint of >40% 6-month PFS rate was met. Hydroxychloroquine is a tolerable autophagy inhibitor in future RCC or other trials.
©2019 American Association for Cancer Research.
Conflict of interest statement
A conflict of interest disclosure statement; RKA is co-inventor of a use patent related to this combination. No revenue has been generated from this intellectual property.
Figures
References
-
- Ghidini M, Petrelli F, Ghidini A, Tomasello G, Hahne JC, Passalacqua R, and Barni S. Clinical development of mTor inhibitors for renal cancer. Expert Opin Investig Drugs. 2017;26(11):1229–37. - PubMed
-
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56. - PubMed
-
- Amaravadi RK, and Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13(24):7271–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous