Tumor-infiltrating human CD4+ regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity
- PMID: 30635355
- PMCID: PMC6685542
- DOI: 10.1126/sciimmunol.aao4310
Tumor-infiltrating human CD4+ regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity
Abstract
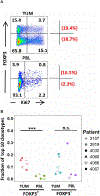
CD4+ regulatory T (Treg) cells have an essential function in maintaining self-tolerance; however, they may also play a detrimental role in antitumor immune responses. The presence of elevated frequencies of Treg cells in tumors correlates with disease progression and poor survival in patients with cancer. The antigen specificity of Treg cells that have expanded in the tumor microenvironment is poorly understood; answering this question may provide important insights for immunotherapeutic approaches. To address this, we used a novel combinatorial approach to characterizing the T cell receptor (TCR) profiles of intratumoral Treg cells from patients with metastatic melanoma, gastrointestinal, and ovarian cancers and elucidated their antigen specificities. The TCR repertoires of tumor-resident Treg cells were diverse yet displayed significant overlap with circulating Treg cells but not with conventional T cells in tumor or blood. TCRs isolated from Treg cells displayed specific reactivity against autologous tumors and mutated neoantigens, suggesting that intratumoral Treg cells act in a tumor antigen-selective manner leading to their activation and clonal expansion in the tumor microenvironment. Tumor antigen-specific Treg-derived TCRs resided in the tumor and in the circulation, suggesting that both Treg cell compartments may serve as a source for tumor-specific TCRs. These findings provide insights into the TCR specificity of tumor-infiltrating human Treg cells that may have potential implications for cancer immunotherapy.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

