Immune responses at the maternal-fetal interface
- PMID: 30635356
- PMCID: PMC6744611
- DOI: 10.1126/sciimmunol.aat6114
Immune responses at the maternal-fetal interface
Abstract
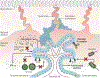
Pregnancy poses an immunological challenge because a genetically distinct (nonself) fetus must be supported within the pregnant female for the required gestational period. Placentation, or the establishment of the fetally derived placenta, is a common strategy used by eutherian mammals to protect the fetus and promote its growth. However, the substantial morphological differences of the placental architecture among species suggest that the process of placentation results from convergent evolution. Although there are considerable similarities in placental function across placental mammals, there are important differences that arise owing to species-specific immunological (and other biological) constraints. This Review focuses on the immunological similarities and differences that occur at the maternal-fetal interface in the context of human and mouse pregnancies. We discuss how the decidua and placenta of these different species form key immunological barriers that sustain maternal tolerance yet generate innate immune responses that prevent microbial infections.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



References
-
- Noyes RW, Hertig AT, Rock J, Dating the Endometrial Biopsy, Fertil. Steril 1, 3–25 (1950). - PubMed
-
- Pijnenborg R, Vercruysse L, Hanssens M, The Uterine Spiral Arteries In Human Pregnancy: Facts and Controversies, Placenta 27, 939–958 (2006). - PubMed
-
- Malassine A, Frendo J-L, Evain-Brion D, A comparison of placental development and endocrine functions between the human and mouse model, Hum. Reprod. Update 9, 531–539 (2003). - PubMed
-
- Tuckey RC, Progesterone synthesis by the human placenta, Placenta 26, 273–281 (2005). - PubMed
-
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E, Uterine Glands Provide Histiotrophic Nutrition for the Human Fetus during the First Trimester of Pregnancy, J Clin Endocrinol Metab 87, 2954–2959 (2002). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

