Heterozygous Tbk1 loss has opposing effects in early and late stages of ALS in mice
- PMID: 30635357
- PMCID: PMC6363427
- DOI: 10.1084/jem.20180729
Heterozygous Tbk1 loss has opposing effects in early and late stages of ALS in mice
Abstract
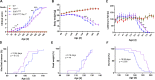
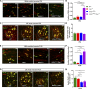
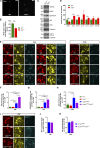
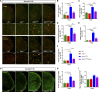
Heterozygous loss-of-function mutations of TANK-binding kinase 1 (TBK1 ) cause familial ALS, yet downstream mechanisms of TBK1 mutations remained elusive. TBK1 is a pleiotropic kinase involved in the regulation of selective autophagy and inflammation. We show that heterozygous Tbk1 deletion alone does not lead to signs of motoneuron degeneration or disturbed autophagy in mice during a 200-d observation period. Surprisingly, however, hemizygous deletion of Tbk1 inversely modulates early and late disease phases in mice additionally overexpressing ALS-linked SOD1G93A , which represents a "second hit" that induces both neuroinflammation and proteostatic dysregulation. At the early stage, heterozygous Tbk1 deletion impairs autophagy in motoneurons and prepones both the clinical onset and muscular denervation in SOD1G93A/Tbk1+/- mice. At the late disease stage, however, it significantly alleviates microglial neuroinflammation, decelerates disease progression, and extends survival. Our results indicate a profound effect of TBK1 on brain inflammatory cells under pro-inflammatory conditions and point to a complex, two-edged role of TBK1 in SOD1-linked ALS.
© 2019 Brenner et al.
Figures


Similar articles
-
The Loss of TBK1 Kinase Activity in Motor Neurons or in All Cell Types Differentially Impacts ALS Disease Progression in SOD1 Mice.Neuron. 2020 Jun 3;106(5):789-805.e5. doi: 10.1016/j.neuron.2020.03.005. Epub 2020 Mar 27. Neuron. 2020. PMID: 32220666
-
Deletion of Tbk1 disrupts autophagy and reproduces behavioral and locomotor symptoms of FTD-ALS in mice.Aging (Albany NY). 2019 Apr 30;11(8):2457-2476. doi: 10.18632/aging.101936. Aging (Albany NY). 2019. PMID: 31039129 Free PMC article.
-
Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis.Proc Natl Acad Sci U S A. 2006 Oct 24;103(43):16021-6. doi: 10.1073/pnas.0607423103. Epub 2006 Oct 16. Proc Natl Acad Sci U S A. 2006. PMID: 17043238 Free PMC article.
-
Functional and structural consequences of TBK1 missense variants in frontotemporal lobar degeneration and amyotrophic lateral sclerosis.Neurobiol Dis. 2022 Nov;174:105859. doi: 10.1016/j.nbd.2022.105859. Epub 2022 Sep 13. Neurobiol Dis. 2022. PMID: 36113750 Review.
-
TBK1: a new player in ALS linking autophagy and neuroinflammation.Mol Brain. 2017 Feb 2;10(1):5. doi: 10.1186/s13041-017-0287-x. Mol Brain. 2017. PMID: 28148298 Free PMC article. Review.
Cited by
-
cGAS and DDX41-STING mediated intrinsic immunity spreads intercellularly to promote neuroinflammation in SOD1 ALS model.iScience. 2022 May 13;25(6):104404. doi: 10.1016/j.isci.2022.104404. eCollection 2022 Jun 17. iScience. 2022. PMID: 35712074 Free PMC article.
-
TBK1 is involved in programmed cell death and ALS-related pathways in novel zebrafish models.Cell Death Discov. 2025 Mar 12;11(1):98. doi: 10.1038/s41420-025-02374-3. Cell Death Discov. 2025. PMID: 40075110 Free PMC article.
-
TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS.Cell. 2020 Oct 29;183(3):636-649.e18. doi: 10.1016/j.cell.2020.09.020. Epub 2020 Oct 7. Cell. 2020. PMID: 33031745 Free PMC article.
-
TDP-43 Proteinopathy Specific Biomarker Development.Cells. 2023 Feb 12;12(4):597. doi: 10.3390/cells12040597. Cells. 2023. PMID: 36831264 Free PMC article. Review.
-
Autophagy Dysfunction in ALS: from Transport to Protein Degradation.J Mol Neurosci. 2022 Jul;72(7):1456-1481. doi: 10.1007/s12031-022-02029-3. Epub 2022 Jun 16. J Mol Neurosci. 2022. PMID: 35708843 Free PMC article. Review.
References
-
- Beers D.R., Henkel J.S., Xiao Q., Zhao W., Wang J., Yen A.A., Siklos L., McKercher S.R., and Appel S.H.. 2006. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 103:16021–16026. 10.1073/pnas.0607423103 - DOI - PMC - PubMed
-
- Boillée S., Yamanaka K., Lobsiger C.S., Copeland N.G., Jenkins N.A., Kassiotis G., Kollias G., and Cleveland D.W.. 2006. Onset and Progression in Inherited ALS Determined by MNs and Microglia. Science. 312:1389–1392. - PubMed
-
- Bonnard M., Mirtsos C., Suzuki S., Graham K., Huang J., Ng M., Itié A., Wakeham A., Shahinian A., Henzel W.J., et al. . 2000. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 19:4976–4985. 10.1093/emboj/19.18.4976 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

