Tau PET imaging in neurodegenerative tauopathies-still a challenge
- PMID: 30635637
- PMCID: PMC6756230
- DOI: 10.1038/s41380-018-0342-8
Tau PET imaging in neurodegenerative tauopathies-still a challenge
Abstract
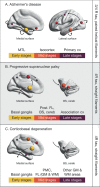
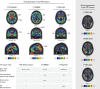
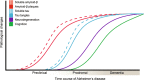
The accumulation of pathological misfolded tau is a feature common to a collective of neurodegenerative disorders known as tauopathies, of which Alzheimer's disease (AD) is the most common. Related tauopathies include progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), Down's syndrome (DS), Parkinson's disease (PD), and dementia with Lewy bodies (DLB). Investigation of the role of tau pathology in the onset and progression of these disorders is now possible due the recent advent of tau-specific ligands for use with positron emission tomography (PET), including first- (e.g., [18F]THK5317, [18F]THK5351, [18F]AV1451, and [11C]PBB3) and second-generation compounds [namely [18F]MK-6240, [18F]RO-948 (previously referred to as [18F]RO69558948), [18F]PI-2620, [18F]GTP1, [18F]PM-PBB3, and [18F]JNJ64349311 ([18F]JNJ311) and its derivative [18F]JNJ-067)]. In this review we describe and discuss findings from in vitro and in vivo studies using both initial and new tau ligands, including their relation to biomarkers for amyloid-β and neurodegeneration, and cognitive findings. Lastly, methodological considerations for the quantification of in vivo ligand binding are addressed, along with potential future applications of tau PET, including therapeutic trials.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. - PubMed
-
- Maas T, Eidenmuller J, Brandt R. Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J Biol Chem. 2000;275:15733–40. - PubMed
-
- Arima K. Ultrastructural characteristics of tau filaments in tauopathies: immuno-electron microscopic demonstration of tau filaments in tauopathies. Neuropathology. 2006;26:475–83. - PubMed
-
- Martin L, Latypova X, Terro F. Post-translational modifications of tau protein: implications for Alzheimer’s disease. Neurochem Int. 2011;58:458–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

