Dynamic [18F]FET-PET/MRI using standard MRI-based attenuation correction methods
- PMID: 30635757
- PMCID: PMC6610265
- DOI: 10.1007/s00330-018-5942-9
Dynamic [18F]FET-PET/MRI using standard MRI-based attenuation correction methods
Abstract
Aim: To assess if tumour grading based on dynamic [18F]FET positron emission tomography/magnetic resonance imaging (PET/MRI) studies is affected by different MRI-based attenuation correction (AC) methods.
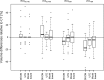
Methods: Twenty-four patients with suspected brain tumours underwent dynamic [18F]FET-PET/MRI examinations and subsequent low-dose computed tomography (CT) scans of the head. The dynamic PET data was reconstructed using the following AC methods: standard Dixon-based AC and ultra-short echo time MRI-based AC (MR-AC) and a model-based AC approach. All data were reconstructed also using CT-based AC (reference). For all lesions and reconstructions, time-activity curves (TACs) and time to peak (TTP) were extracted using different region-of-interest (ROI) and volume-of-interest (VOI) definitions. According to the most common evaluation approaches, TACs were categorised into two or three distinct curve patterns. Changes in TTP and TAC patterns compared to PET using CT-based AC were reported.
Results: In the majority of cases, TAC patterns did not change. However, TAC pattern changes as well as changes in TTP were observed in up to 8% and 17% of the cases when using different MR-AC methods and ROI/VOI definitions, respectively. However, these changes in TTP and TAC pattern were attributed to different delineations of the ROIs/VOIs in PET corrected with different AC methods.
Conclusion: PET/MRI using different MR-AC methods can be used for the assessment of TAC patterns in dynamic [18F]FET studies, as long as a meaningful delineation of the area of interest within the tumour is ensured.
Key points: • PET/MRI using different MR-AC methods can be used for dynamic [18F]FET studies. • A meaningful segmentation of the area of interest needs to be ensured, mandating a visual validation of the delineation by an experienced reader.
Keywords: Brain neoplasms; Magnet resonance imaging; Positron emission tomography; Radionuclide imaging.
Conflict of interest statement
Matthias Fenchel is an employee of Siemens Healthcare GmbH.
Figures




Similar articles
-
Multi-Atlas-Based Attenuation Correction for Brain 18F-FDG PET Imaging Using a Time-of-Flight PET/MR Scanner: Comparison with Clinical Single-Atlas- and CT-Based Attenuation Correction.J Nucl Med. 2016 Aug;57(8):1258-64. doi: 10.2967/jnumed.115.169045. Epub 2016 Mar 24. J Nucl Med. 2016. PMID: 27013697
-
PET/MRI for Oncologic Brain Imaging: A Comparison of Standard MR-Based Attenuation Corrections with a Model-Based Approach for the Siemens mMR PET/MR System.J Nucl Med. 2017 Sep;58(9):1519-1525. doi: 10.2967/jnumed.116.186148. Epub 2017 Mar 2. J Nucl Med. 2017. PMID: 28254872
-
Combined PET/MR imaging in neurology: MR-based attenuation correction implies a strong spatial bias when ignoring bone.Neuroimage. 2014 Jan 1;84:206-16. doi: 10.1016/j.neuroimage.2013.08.042. Epub 2013 Aug 29. Neuroimage. 2014. PMID: 23994317
-
Hybrid Positron Emission Tomography/Magnetic Resonance Imaging: Challenges, Methods, and State of the Art of Hardware Component Attenuation Correction.Invest Radiol. 2016 Oct;51(10):624-34. doi: 10.1097/RLI.0000000000000289. Invest Radiol. 2016. PMID: 27175550 Review.
-
Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques.Eur J Nucl Med Mol Imaging. 2009 Mar;36 Suppl 1:S93-104. doi: 10.1007/s00259-008-1007-7. Eur J Nucl Med Mol Imaging. 2009. PMID: 19104810 Review.
Cited by
-
Impact of 18F-FET PET/MRI on Clinical Management of Brain Tumor Patients.J Nucl Med. 2022 Apr;63(4):522-527. doi: 10.2967/jnumed.121.262051. Epub 2021 Aug 5. J Nucl Med. 2022. PMID: 34353870 Free PMC article.
-
Take Advantage of Glutamine Anaplerosis, the Kernel of the Metabolic Rewiring in Malignant Gliomas.Biomolecules. 2020 Sep 26;10(10):1370. doi: 10.3390/biom10101370. Biomolecules. 2020. PMID: 32993063 Free PMC article. Review.
-
Update on the Use of PET/MRI Contrast Agents and Tracers in Brain Oncology: A Systematic Review.Int J Nanomedicine. 2022 Jul 29;17:3343-3359. doi: 10.2147/IJN.S362192. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35937076 Free PMC article.
-
Impact of improved dead time correction on the quantification accuracy of a dedicated BrainPET scanner.PLoS One. 2024 Apr 5;19(4):e0296357. doi: 10.1371/journal.pone.0296357. eCollection 2024. PLoS One. 2024. PMID: 38578749 Free PMC article.
-
DeepDixon synthetic CT for [18F]FET PET/MRI attenuation correction of post-surgery glioma patients with metal implants.Front Neurosci. 2023 Apr 6;17:1142383. doi: 10.3389/fnins.2023.1142383. eCollection 2023. Front Neurosci. 2023. PMID: 37090806 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

