Zinc Deficiency and Arsenic Exposure Can Act Both Independently or Cooperatively to Affect Zinc Status, Oxidative Stress, and Inflammatory Response
- PMID: 30635848
- PMCID: PMC6625954
- DOI: 10.1007/s12011-019-1631-z
Zinc Deficiency and Arsenic Exposure Can Act Both Independently or Cooperatively to Affect Zinc Status, Oxidative Stress, and Inflammatory Response
Abstract
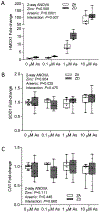
The negative health impact of zinc deficiency overlaps significantly with arsenic exposure, and is associated with increased risk for chronic diseases. Arsenic contamination in the groundwater often co-exists in regions of the world that are prone to zinc deficiency. Notably, low zinc status shares many hallmarks of arsenic exposure, including increased oxidative stress and inflammation. Despite their common targets and frequent co-distribution in the population, little is known regarding the interaction between zinc deficiency and arsenic exposure. In this study, we tested the effect of arsenic exposure at environmentally relevant doses in combination with a physiologically relevant level of zinc deficiency (marginal zinc deficiency) on zinc status, oxidative damage, and inflammation. In cell culture, zinc-deficient THP-1 monocytes co-exposed with arsenic resulted in further reduction in intracellular zinc, as well as further increase in oxidative stress and inflammatory markers. In an animal study, zinc-deficient mice had further decrease in zinc status when co-exposed to arsenic. Zinc deficiency, but not arsenic exposure, resulted in an increase in baseline transcript abundance of inflammatory markers in the liver. Upon lipopolysaccharide challenge to elicit an acute inflammatory response, arsenic exposure, but not zinc deficiency, resulted in an increase in proinflammatory response. In summary, zinc deficiency and arsenic exposure can function independently or cooperatively to affect zinc status, oxidant stress, and proinflammatory response. The results highlight the need to consider both nutritional status and arsenic exposures together when considering their impact on health outcomes in susceptible populations.
Keywords: Arsenic; Inflammation; Oxidative stress; Zinc.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Figures

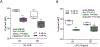



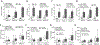
References
-
- Khansari N, Shakiba Y, Mahmoudi M (2009) Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov 3 (1):73–80 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

